Details of the Drug
General Information of Drug (ID: DM1LGKP)
| Drug Name |
Mevastatin
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Mevastatin; compactin; 73573-88-3; ML-236B; Mevastatinum; Mevastatine; Mevastatina; Mevastatinum [INN-Latin]; Antibiotic ML 236B; Mevastatin [INN]; CS 500; Compactin (penicillium); Mevastatine [INN-French]; UNII-1UQM1K0W9X; ML 236 B; Mevastatina [INN-Spanish]; CCRIS 4505; 1UQM1K0W9X; CHEMBL54440; CHEBI:34848; [(1S,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-7-methyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] (2S)-2-methylbutanoate; Mevastatin (Compactin); ML236B
|
|||||
| Affected Organisms |
Humans and other mammals
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Structure |
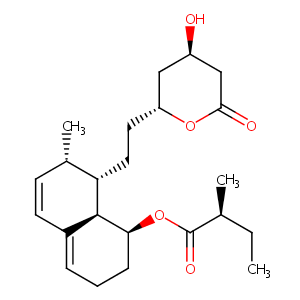 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 390.5 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 3.9 | |||||
| Rotatable Bond Count (rotbonds) | 7 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | |||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
References
