Details of the Drug
General Information of Drug (ID: DM1PV4Y)
| Drug Name |
CS-600G
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Koloxo; Loxoprofen; Loxoprofen (INN); Loxoprofen [INN]; Loxoprofen sodium hydrate; Loxoprofene; Loxoprofene [French]; Loxoprofeno; Loxoprofeno [Spanish]; Loxoprofenum; Loxoprofenum [Latin]; YMBXTVYHTMGZDW-UHFFFAOYSA-N; sodium loxoprofen; yl]-propionic acid; 2-(4-((2-Oxocyclopentyl)methyl)phenyl)propanoic acid; 2-[4-(2-Oxo-cyclopentylmethyl)-phen; 2-[4-[(2-oxocyclopentyl)methyl]phenyl]propanoic acid; 2-{4-[(2-oxocyclopentyl)methyl]phenyl}propanoic acid; 68767-14-6; CHEBI:76172; CHEMBL19299; CS-600; MFCD00864331; Oxeno
|
|||||
| ATC Code | ||||||
| Structure |
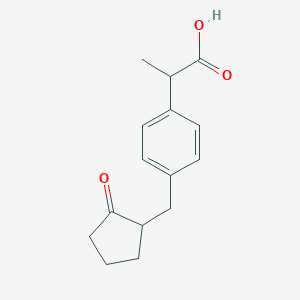 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 246.3 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 2.4 | |||||
| Rotatable Bond Count (rotbonds) | 4 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
References
