Details of the Drug
General Information of Drug (ID: DM2RX0I)
| Drug Name |
Abametapir
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
5,5'-Dimethyl-2,2'-bipyridine; 1762-34-1; 5,5'-Dimethyl-2,2'-dipyridyl; 5,5'-Dimethyl-2,2'-bipyridyl; 6,6'-Bi-3-picoline; 2,2'-BIPYRIDINE, 5,5'-DIMETHYL-; HA-44; 6,6'-Di-3-picolyl; 6,6'-Di-3-picoline; UNII-6UO390AMFB; 5-methyl-2-(5-methylpyridin-2-yl)pyridine; MFCD01740554; 6UO390AMFB; CHEMBL2205807; 5,5'-dimethyl-2,2'-bipyridinyl; 5,5 -Dimethyl-2,2 -bipyridine; Xeglyze; BRN 0123183; Abametapir [USAN:INN]; Xeglyze(Abametapir); Xeglyze (TN); PubChem24353; Abametapir (USAN/INN); ACMC-209eb4; SCHEMBL351152; HA44; 2,2 -Bis-(5-methylpyridyl); DTXSID00170095; ZINC403335; 9238AA; ANW-22814; BDBM50401351; LT0042; s5752; 5,5''-Dimethyl-2,2''-bipyridine; AKOS005257775; CS-W004546; DB11932; MCULE-8581798506; SB17220; 5,5 inverted exclamation marka-Dimethyl-2,2 inverted exclamation marka-dipyridyl; 5,5'-Dimethyl-2,2'-dipyridyl, 98%; AK-63331; DS-15219; SY052805; FT-0689891; D10687; W-108621; Q27265547; 5,5 inverted exclamation mark -Dimethyl-2,2 inverted exclamation mark -bipyridyl
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Pediculus humanus
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
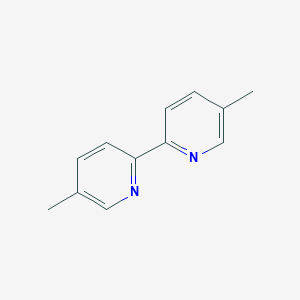 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 184.24 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Abametapir (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | ||||
|---|---|---|---|---|---|
| 2 | FDA Approved Drug Products: Xeglyze (Abametapir) topical lotion | ||||
| 3 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 4 | Product Information. Gavreto (pralsetinib). Blueprint Medicines Corporation, Cambridge, MA. | ||||
