Details of the Drug
General Information of Drug (ID: DM3Q1SM)
| Drug Name |
obeticholic acid
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | INT747; INT-747; 6-ethylchenodeoxycholic acid; 6-ECDCA; OCA; 6-Et CDCA | ||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
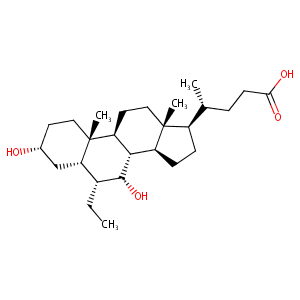 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 420.6 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.7 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Primary biliary cholangitis | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | DB96.1 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from obeticholic acid (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | 2016 FDA drug approvals. Nat Rev Drug Discov. 2017 Feb 2;16(2):73-76. | ||||
|---|---|---|---|---|---|
| 2 | Nuclear receptors and lipid physiology: opening the X-files. Science. 2001 Nov 30;294(5548):1866-70. | ||||
| 3 | Product Monograph: Ocaliva (obeticholic acid) oral tablets | ||||
| 4 | FDA Approved Products: Ocaliva (obeticholic acid) oral tablets | ||||
| 5 | NIH InXight Drugs: Obeticholic acid | ||||
| 6 | Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J Biol Chem. 1994 Jul 29;269(30):19375-9. | ||||
| 7 | Pharmacotoxicology of clinically-relevant concentrations of obeticholic acid in an organotypic human hepatocyte system. Toxicol In Vitro. 2017 Mar;39:93-103. | ||||
| 8 | Product Information. Nexletol (bempedoic acid). Esperion Therapeutics, Ann Arbor, MI. | ||||
| 9 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 10 | Product Information. Vosevi (sofosbuvir/velpatasvir/voxilaprevir). Gilead Sciences, Foster City, CA. | ||||
| 11 | Product Information. Hetlioz (tasimelteon). Vanda Pharmaceuticals Inc, Rockville, MD. | ||||
| 12 | Product Information. Saphris (asenapine). Schering-Plough Corporation, Kenilworth, NJ. | ||||
| 13 | Product Information. Ocaliva (obeticholic acid). Intercept Pharmaceuticals, Inc., New York, NY. | ||||
