Details of the Drug
General Information of Drug (ID: DMMSDOY)
| Drug Name |
Stiripentol
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | Diacomit; ME-2080; Stiripentol (oral); Stiripentol (oral), Laboratoires Biocodex | ||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
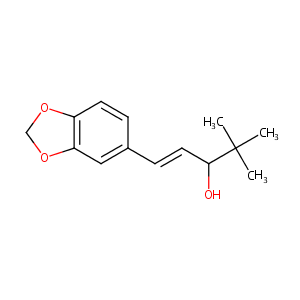 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 234.29 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.6 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Stiripentol (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | The effects of stiripentol on GABA(A) receptors.Epilepsia. 2011 Apr;52 Suppl 2:76-8. | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5469). | ||||
| 3 | Serrano-Rodriguez JM, Gomez-Diez M, Esgueva M, Castejon-Riber C, Mena-Bravo A, Priego-Capote F, Ayala N, Caballero JMS, Munoz A: Pharmacokinetic/pharmacodynamic modeling of benazepril and benazeprilat after administration of intravenous and oral doses of benazepril in healthy horses. Res Vet Sci. 2017 Oct;114:117-122. doi: 10.1016/j.rvsc.2017.03.016. Epub 2017 Mar 28. | ||||
| 4 | European Medicines Agency (EMA): DIACOMIT Summary of Product Characteristics | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Trojnar MK, Wojtal K, Trojnar MP, Czuczwar SJ: Stiripentol. A novel antiepileptic drug. Pharmacol Rep. 2005 Mar-Apr;57(2):154-60. | ||||
| 7 | Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABA-A receptor channels. Epilepsia. 2006 Apr;47(4):704-16. | ||||
| 8 | Product characteristics of Diacomit. | ||||
| 9 | FDA Label of Diacomit. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 10 | Stiripentol. Expert Opin Investig Drugs. 2005 Jul;14(7):905-11. | ||||
| 11 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 12 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 13 | Product Information. Olinvyk (oliceridine). Trevena Inc, Chesterbrook, PA. | ||||
| 14 | Abbas R, Hug BA, Leister C, Burns J, Sonnichsen D "Pharmacokinetics of oral neratinib during co-administration of ketoconazole in healthy subjects." Br J Clin Pharmacol 71 (2011): 522-7. [PMID: 21395644] | ||||
| 15 | Product Information. Verzenio (abemaciclib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 16 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 17 | Product Information. Rexulti (brexpiprazole). Otsuka American Pharmaceuticals Inc, Rockville, MD. | ||||
| 18 | Product Information. Polivy (polatuzumab vedotin). Genentech, South San Francisco, CA. | ||||
| 19 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 20 | Product Information. Pifeltro (doravirine). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 21 | Canadian Pharmacists Association. | ||||
| 22 | Product Information. Viberzi (eluxadoline). Actavis Pharma, Inc., Parsippany, NJ. | ||||
| 23 | Product Information. Gavreto (pralsetinib). Blueprint Medicines Corporation, Cambridge, MA. | ||||
| 24 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 25 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 26 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 27 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 28 | Product Information. Nurtec ODT (rimegepant). Biohaven Pharmaceuticals, New Haven, CT. | ||||
| 29 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 30 | Product Information. Nourianz (istradefylline). Kyowa Kirin, Inc, Bedminster, NJ. | ||||
| 31 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
| 32 | Product Information. Rinvoq (upadacitinib). AbbVie US LLC, North Chicago, IL. | ||||
| 33 | Product Information. Odomzo (sonidegib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 34 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
