| Drug Name |
Asacolitin
|
| Synonyms |
Apriso; Asacol; Asacol HD; 5-Aminosalicylic acid; Benzoic acid, 5-amino-2-hydroxy-; Canasa; Claversal; Fisalamine; Lialda; Lixacol; Mesalamine [USAN]; Mesalazina; Mesalazina [Spanish]; Mesalazine; Mesalazinum; Mesalazinum [Latin]; Mesasal; Pentasa; Rowasa; Salofalk; m-Aminosalicylic acid; mesalamine; p-Aminosalicylsaeure; p-Aminosalicylsaeure [German]; sfRowasa; 2-Hydroxy-5-aminobenzoic acid; 5-ASA; 5-Amino Salicylic Acid; 5-Amino-2-hydroxybenzoic acid; 5-amino-2-hydroxy-benzoic acid; 89-57-6
|
| Affected Organisms |
Humans and other mammals
|
| ATC Code |
- A07EC02: Asacolitin
- A07EC: Aminosalicylic acid and similar agents
- A07E: INTESTINAL ANTIINFLAMMATORY AGENTS
- A07: ANTIDIARRHEALS, INTESTINAL ANTIINFLAMMATORY/ANTIINFECTIVE AGENTS
- A: ALIMENTARY TRACT AND METABOLISM
- A07EC02: Asacolitin
- A07EC: Aminosalicylic acid and similar agents
- A07E: INTESTINAL ANTIINFLAMMATORY AGENTS
- A07: ANTIDIARRHEALS, INTESTINAL ANTIINFLAMMATORY/ANTIINFECTIVE AGENTS
- A: ALIMENTARY TRACT AND METABOLISM
|
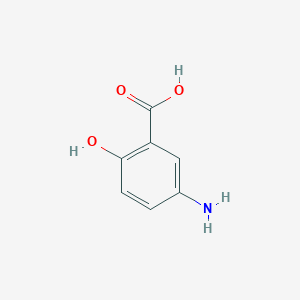
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
153.14 |
|
| Logarithm of the Partition Coefficient (xlogp) |
1.3 |
| Rotatable Bond Count (rotbonds) |
1 |
| Hydrogen Bond Donor Count (hbonddonor) |
3 |
| Hydrogen Bond Acceptor Count (hbondacc) |
4 |
| ADMET Property |
- Elimination
-
N-acetylmesalazine was the primary compound excreted in the urine (19% to 30%) following the controlled-release dosing
[]
- Half-life
-
The concentration or amount of drug in body reduced by one-half in 7 - 12 hours
[1]
- Vd
-
The volume of distribution (Vd) of drug is 0.2 L/kg
[1]
|
| Chemical Identifiers |
- Formula
- C7H7NO3
- IUPAC Name
5-amino-2-hydroxybenzoic acid - Canonical SMILES
-
C1=CC(=C(C=C1N)C(=O)O)O
- InChI
-
KBOPZPXVLCULAV-UHFFFAOYSA-N
- InChIKey
-
1S/C7H7NO3/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3,9H,8H2,(H,10,11)
|
| Cross-matching ID |
- PubChem CID
- 4075
- ChEBI ID
-
- CAS Number
-
- UNII
-
- DrugBank ID
-
- INTEDE ID
- DR1967
|
|
|
|
|
|
|
|



