Details of the Drug
General Information of Drug (ID: DM5GQD7)
| Drug Name |
Metoprine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
METOPRINE; Methodichlorophen; DDMP; 7761-45-7; Metoprine [USAN]; BW-197U; TCMDC-123931; NSC-19494; SK 5265; 5-(3,4-DICHLOROPHENYL)-6-METHYLPYRIMIDINE-2,4-DIAMINE; U 197; NSC7364; U-197; UNII-2L9RKX796Q; Metoprine (USAN); BW 50-197; METOPRINE, METHODICHLOROPHEN; 5-(3,4-Dichlorophenyl)-6-methyl-2,4-pyrimidinediamine; NSC 7364; 2,4-Pyrimidinediamine, 5-(3,4-dichlorophenyl)-6-methyl-; NSC 19494; BW50197; BRN 0223622; 5-(3,4-Dichlorphenyl)-6-methyl-2,4-pyrimidindiamin; 2,4-Diamino-5-(3,4-dichlorophenyl)-6-methylpyrimidine; MLS002701892; DDMP; BW 197 U; BW 1970; BW 197U; BW 50197; BW 50,197; 2,4-Diamino-5-(3',4'-dichlorophenyl)-6-methyl pyrimidine; Chloroaniline 1
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
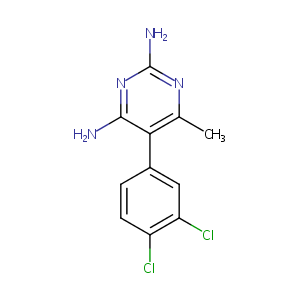 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 269.13 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Advanced cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A00-2F9Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
