Details of the Drug
General Information of Drug (ID: DM7YNV9)
| Drug Name |
Iclaprim
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
192314-93-5; AR-100; 5-[(2-cyclopropyl-7,8-dimethoxy-2h-chromen-5-yl)methyl]pyrimidine-2,4-diamine; RO-48-2622; Mersarex; 5-((2-Cyclopropyl-7,8-dimethoxy-2H-chromen-5-yl)methyl)pyrimidine-2,4-diamine; Iclaprim [USAN:INN]; Iclaprim (USAN/INN); 2,4-Pyrimidinediamine, 5-((2-cyclopropyl-7,8-dimethoxy-2H-1-benzopyran-5-yl)methyl)-; 2,4-Pyrimidinediamine, 5-[(2-cyclopropyl-7,8-dimethoxy-2H-1-benzopyran-5-yl)methyl]-; AR 100; AC1Q4WM5; SCHEMBL379386; CHEMBL134561; AC1L4U54; SCHEMBL12899446; CTK4E0971; BDBM18070; Iclaprim [INN]; AR-102; AR-100001; 5-((2RS)-2-cyclopropyl-7,8-dimethoxy-2H-chromen-5-ylmethyl)pyrimidine-2,4-diamine
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
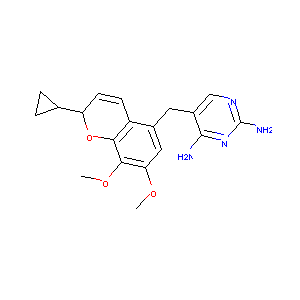 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 354.4 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.5 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
