Details of the Drug
General Information of Drug (ID: DM82JP0)
| Drug Name |
Lynestrenol
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Linestrenol; Linestrenol [INN-Spanish]; Linestrenolo [DCIT]; Lynenol; Lynestrenolum [INN-Latin]; Lynoestrenol; Lynoestrenol [Progestins]; Lynstranol; Endometril; Ethinyl oestrenol; Ethinylestrenol; Ethinyloestranol; Ethynloestrenol; Ethynylestrenol; Exluten; Exlution; Exluton; Exlutona; IND 1006; LYNESTRENOL; Org 485-50; Orgametil; Orgametril; Orgametrol; 17-alpha-Ethynylestrenol; 17-alpha-Ethynyloestrenol; 17alpha-Ethynylestrenol; 3-Desoxynorlutin; 52-76-6; CCRIS 9093; EINECS 200-151-4; NSC 37725; NSC-37725; UNII-N2Z8ALG4U5
|
|||||
| ATC Code |
|
|||||
| Structure |
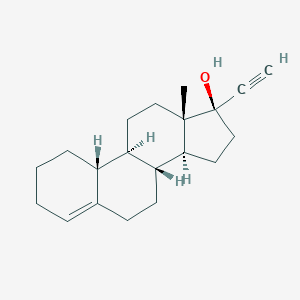 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 284.4 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 4.5 | |||||
| Rotatable Bond Count (rotbonds) | 1 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | |||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
