Details of the Drug
General Information of Drug (ID: DM9AGWQ)
| Drug Name |
HEXESTROL
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
hexestrol; Dihydrodiethylstilbestrol; Vitestrol; Hexoestrolum; Exestrol; 5635-50-7; Stilbestrol, dihydro-; 4,4'-(1,2-Diethylethylene)diphenol; 4,4'-(hexane-3,4-diyl)diphenol; Synoestrolum; Hexanestrol; Cycloestrol; Esestrolo [DCIT]; Phenol, 4,4'-(1,2-diethyl-1,2-ethanediyl)bis-; Hexestrolum [INN-Latin]; Hexane, 3,4-bis(4-hydroxyphenyl)-; EINECS 227-082-2; 3,4-Bis(p-hydroxyphenyl)hexane; Hormoestrol; Syntrogene; Synthovo; Phenol, 4,4'-(1,2-diethylethylene)di-; CHEBI:31669; PBBGSZCBWVPOOL-UHFFFAOYSA-N; Hexestrol [INN]
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
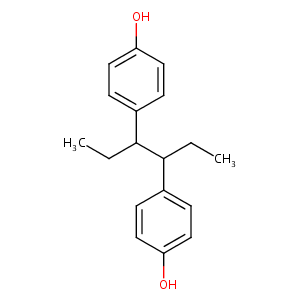 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 270.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
