Details of the Drug
General Information of Drug (ID: DM9SCME)
| Drug Name |
IRX4204
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
220619-73-8; CHEMBL75133; UNII-877M97Z38Y; VTP-194204; 877M97Z38Y; KB-145960; SCHEMBL3437269; MolPort-042-665-869; ZINC1550770; IRX-4204; 3-Methyl-5-[2-methyl-2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-naphthalen-2-yl)-cyclopropyl]-penta-2,4-dienoic acid; BDBM50101445; DB11806; VTP 194204; (+)-VTP-194204; AGN 4204; (2E,4E)-3-Methyl-5-[(1S,2S)-2-methyl-2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-naphthalen-2-yl)-cyclopropyl]-penta-2,4-dienoic acid
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
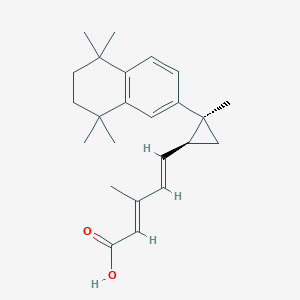 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 352.5 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 7.3 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
