Details of the Drug
General Information of Drug (ID: DMANEC9)
| Drug Name |
Esculin
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Aesculetin glukosid; Aesculinum; Esculetin 6-O-glucoside; Esculetin 6-beta-D-glucoside; Esculin; Esculin hydrate; Esculin hydrate, 97%; Esculine; Esculoside; Polychrome; Venoplant; XHCADAYNFIFUHF-TVKJYDDYSA-N; aesculin; (-)-Esculin; 1Y1L18LQAF; 531-75-9; 6,7-Dihydroxycoumarin 6-glucoside; 6,7-Dihydroxycoumarin-6-O-glucoside; 6-(beta-D-Glucopyranosyloxy)-7-hydroxy-2H-1-benzopyran-2-one; 6-(beta-D-Glucopyranosyloxy)-7-hydroxy-cumarin; 7-Hydroxy-6-cumarinyl-glucosid; 7-Hydroxy-6-glucosyloxy-2H-chromene; CHEBI:4853; UNII-1Y1L18LQAF
|
|||||
| Affected Organisms |
Aerococcus viridans
|
|||||
| Structure |
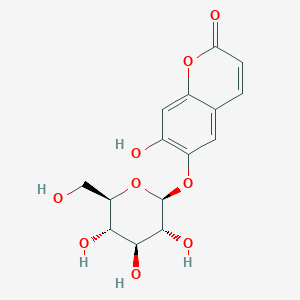 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 340.28 | ||||
| Logarithm of the Partition Coefficient (xlogp) | -0.6 | |||||
| Rotatable Bond Count (rotbonds) | 3 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
References
