Details of the Drug
General Information of Drug (ID: DMB0OZ3)
| Drug Name |
PF-429242
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
947303-87-9; PF-429242; (R)-4-((Diethylamino)methyl)-N-(2-methoxyphenethyl)-N-(pyrrolidin-3-yl)benzamide; UNII-49WB3OA7VN; 49WB3OA7VN; PF 429242; PF429242; CHEMBL233611; J-502224; C25H35N3O2; 4-[(Diethylamino)methyl]-N-[2-(2-methoxyphenyl)ethyl]-N-(3R)-3-pyrrolidinylbenzamide; (R)-4-((Diethylamino)methyl)-N-(2-methoxyphenethyl)-N-(pyrrolidin-3-yl)benz amide; (-)-PF-429242; SCHEMBL2742702; CTK8B5018; DTXSID00635376; EX-A1409; ANW-47048; ZINC28823615; BDBM50216181; AKOS015998619; RL05965; Benzamide, 4-((diethylamino)methyl)-N-(2-(
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
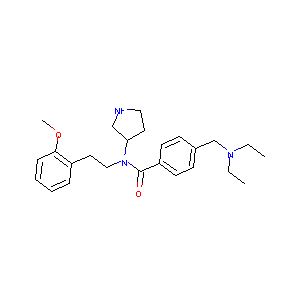 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 409.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
References
