Details of the Drug
General Information of Drug (ID: DMBAWDG)
| Drug Name |
Thiolactomycin
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Antibiotic 2-200; 82079-32-1; (R)-(+)-Thiolactomycin; (+)-Thiolactomycin; BRN 4423670; 4-HYDROXY-3,5-DIMETHYL-5-(2-METHYL-BUTA-1,3-DIENYL)-5H-THIOPHEN-2-ONE; 2(5H)-Thiophenone, 3,5-dimethyl-4-hydroxy-5-(2-methyl-1,3-butadienyl)-, (R-(E))-; (R-(E))-3,5-Dimethyl-4-hydroxy-5-(2-methyl-1,3-butadienyl)-2(5H)-thiophenone; TLM; 2(5H)-Thiophenone, 4-hydroxy-3,5-dimethyl-5-(2-methyl-1,3-butadienyl)-, (S-(E))-; (2R)-5-hydroxy-2,4-dimethyl-2-[(1E)-2-methylbuta-1,3-dienyl]thiophen-3-one; (5R)-Thiolactomycin; 2vb8
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
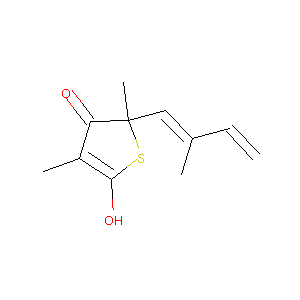 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 210.29 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
References
