| Drug Name |
RUPATADINE
|
| Synonyms |
Rupatadine trihydrochloride; UNII-G61C8NZY2T; G61C8NZY2T; 156611-76-6; Rupatadine HCl; Rupatadine hydrochloride; UR 12592; AC1L320L; DTXSID90166109; BQFOTHHRVVHLEW-UHFFFAOYSA-N; 8-Chloro-6,11-dihydro-11-(1-((5-methyl-3-pyridinyl)methyl)-4-piperidinylidene)-5H-benzo(5,6)cyclohepta(1,2-b)pyridine trihydrochloride; 5H-Benzo(5,6)cyclohepta(1,2-b)pyridine, 8-chloro-6,11-dihydro-11-(1-((5-methyl-3-pyridinyl)methyl)-4-piperidinylidene)-, trihydrochloride
|
| ATC Code |
- R06AX28: RUPATADINE
- R06AX: Other antihistamines for systemic use
- R06A: ANTIHISTAMINES FOR SYSTEMIC USE
- R06: ANTIHISTAMINES FOR SYSTEMIC USE
- R: RESPIRATORY SYSTEM
|
| Drug Type |
Small molecular drug
|
| Structure |
|
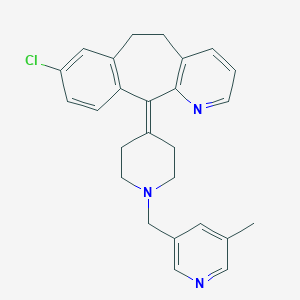 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 1 |
Molecular Weight (mw) |
416 |
|
| Logarithm of the Partition Coefficient (xlogp) |
5.8 |
| Rotatable Bond Count (rotbonds) |
2 |
| Hydrogen Bond Donor Count (hbonddonor) |
0 |
| Hydrogen Bond Acceptor Count (hbondacc) |
3 |
| ADMET Property |
- Absorption
-
The drug is rapidly absorbed and reaches peak plasma concentration in 1 hours
[]
- Clearance
-
The sytemic clearance of drug is 1556.2 L/h
[]
- Half-life
-
The concentration or amount of drug in body reduced by one-half in 15.9 hours (in children 2 - 5 years old), 12.3 hours (in children 6 - 11 years old), 5.9 hours (in adults), and 8.7 hours (in geriatric patients)
[]
- Metabolism
-
The drug is metabolized via oxidation mediated primarily by CYP3A4
[]
- Vd
-
The volume of distribution (Vd) of drug is 9799 L
[]
|
| Chemical Identifiers |
- Formula
- C26H26ClN3
- IUPAC Name
13-chloro-2-[1-[(5-methylpyridin-3-yl)methyl]piperidin-4-ylidene]-4-azatricyclo[9.4.0.03,8]pentadeca-1(11),3(8),4,6,12,14-hexaene - Canonical SMILES
-
CC1=CC(=CN=C1)CN2CCC(=C3C4=C(CCC5=C3N=CC=C5)C=C(C=C4)Cl)CC2
- InChI
-
InChI=1S/C26H26ClN3/c1-18-13-19(16-28-15-18)17-30-11-8-20(9-12-30)25-24-7-6-23(27)14-22(24)5-4-21-3-2-10-29-26(21)25/h2-3,6-7,10,13-16H,4-5,8-9,11-12,17H2,1H3
- InChIKey
-
WUZYKBABMWJHDL-UHFFFAOYSA-N
|
| Cross-matching ID |
- PubChem CID
- 133017
- ChEBI ID
-
- CAS Number
-
- UNII
-
- DrugBank ID
-
- TTD ID
- D0S1CQ
- INTEDE ID
- DR1451
|
|
|
|
|
|
|
|



