Details of the Drug
General Information of Drug (ID: DMDHGM2)
| Drug Name |
MAACKIAIN
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Maackiain; (-)-Maackiain; Inermin; Inermine; 2035-15-6; L-Maackiain; CHEBI:99; UNII-TF360D25IJ; (6ar,12ar)-6a,12a-dihydro-6h-[1,3]dioxolo[5,6][1]benzofuro[3,2-c]chromen-3-ol; TF360D25IJ; HUKSJTUUSUGIDC-ZBEGNZNMSA-N; 19908-48-6; 3-Hydroxy-8,9-methylenedioxypterocarpan; ST077155; (6aR,12aR)-3-hydroxy-8,9-methylenedioxypterocarpane; (6aR,12aR)-6a,12a-Dihydro-6H-[1,3]dioxolo[4',5':5,6]benzofuro[3,2-c]chromen-3-ol; Maackiaine; Trifolirhizin aglycone; (+/-)-Maackiain; AC1Q70VV; CHEMBL334918; AC1L3M84; (-)-(6aR,12aR)-maackiain
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
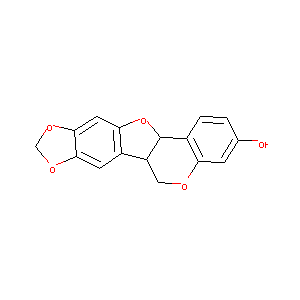 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 284.26 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
