Details of the Drug
General Information of Drug (ID: DMGTSCP)
| Drug Name |
JWH-015
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
JWH-015; 155471-08-2; JWH 015; UNII-W4FL204T10; (2-Methyl-1-propyl-1H-indol-3-yl)-1-naphthalenylmethanone; JWH015; (2-methyl-1-propylindol-3-yl)-naphthalen-1-ylmethanone; CHEMBL306764; W4FL204T10; MFCD02094630; (2-methyl-1-propyl-1H-indol-3-yl)(naphthalen-1-yl)methanone; JHW 015; SR-01000597570; (2-Methyl-1-propylindol-3-yl)(naphthalen-1-yl)methanone; NCGC00025117-01; Tocris-1341; PubChem19328; MLS002153392; SCHEMBL497044; GTPL5558; AC1N784D; BDBM21282; CHEBI:92318; DTXSID60165902; AOB5643; MolPort-003-983-602
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
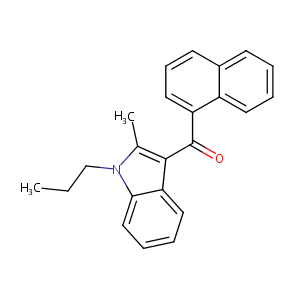 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 327.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Organ transplant rejection | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | NE84 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
