Details of the Drug
General Information of Drug (ID: DMHRG8Q)
| Drug Name |
Carbidopa
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Atamet; Carbidopum; Lodosin; Lodosyn; Methyldopahydrazine; Stalevo; Carbidopa Monohydrate; Carbidopa anhydrous; Carbidopa hydrate; Carbidopum monohydricum; MK 486; Alpha-Methyldopahydrazine; C-126; C-DOPA; Carbidopa (anhydrous); Carbidopum [INN-Latin]; Lodosyn (TN); Lodosyn, Carbidopa; MK-485; MK-486; N-Aminomethyldopa; Carbidopa [USAN:INN:BAN]; Carbidopa-1-wasser; Hadrazino-alpha-methyldopa; L-alpha-Methyldopahydrazine; Carbidopa, (S)-Isomer; Carbidopa, Entacapone, & Levodopa; S(-)-CARBIDOPA; S-(-)-Carbidopa; L-alpha-(3,4-dihydroxybenzyl)-alpha-hydrazinopropionic acid monohydrate; Alpha-Hydrazino-alpha-methyl-beta-(3,4-dihydroxyphenyl)propionic acid; L-3-(3,4-Dihydroxyphenyl)-2-methyl-2-hydrazinopropionic acid; L-alpha-Methyl-alpha-hydrazino-beta-(3,4-dihydroxyphenylpropionic acid; L-alpha-Methyl-beta-(3,4-dihydroxyphenyl)-alpha-hydrazinopropionic acid; S(-)-alpha-Hydrazino-3,4-dihydroxy-alpha-methylhydrocinnamic acid monohydrate; S-(-)-alpha-Hydrazino-3,4-dihydroxy-2-methylbenzenepropanoic acid; Benzenepropanoic acid, alpha-hydrazino-3,4-dihydroxy-alpha-methyl-, monohydrate, (S); KINSON, 3-(3,4-DIHYDROXY-PHENYL)-2-HYDRAZINO-2-METHYL-PROPIONIC ACID; Hydrocinnamic acid, (-)-L-alpha-hydrazino-3,4-dihydroxy-alpha-methyl-, monohydrate; (-)-L-alpha-Hydrazino-3,4-dihydroxy-alpha-methylhydrocinnamic acid; (-)-L-alpha-Hydrazino-3,4-dihydroxy-alpha-methylhydrocinnamic acid monohydrate; (2S)-3-(3,4-dihydroxyphenyl)-2-hydrazino-2-methylpropanoic acid; (2S)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic acid; (2S)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic acid hydrate; (2S)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic acid monohydrate; (2S)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic acid-water (1/1); (S)-(-)-carbidopa; (S)-(-)-carbidopa hydrate; (S)-alpha-Hydrazino-3,4-dihydroxy-alpha-methyl-benzenepropanoic acid monohydrate; (S)-carbidopa; (S)-carbidopahydrate; (alphaS)-alpha-hydrazino-3,4-dihydroxy-alpha-methylbenzenepropanoic acid; (alphaS)-alpha-hydrazino-3,4-dihydroxy-alpha-methylbenzenepropanoic acid monohydrate
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiparkinson Agents
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
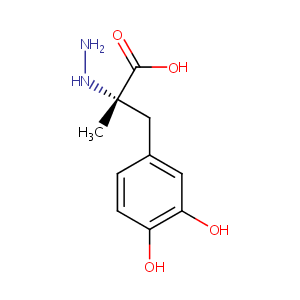 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 226.23 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2.2 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Parkinson disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A00.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Carbidopa (Comorbidity)
|
|||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5159). | ||||
|---|---|---|---|---|---|
| 2 | Carbidopa FDA Label | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Chana P, Fierro A, Reyes-Parada M, Saez-Briones P: [Pharmacokinetic comparison of Sinemet and Grifoparkin (levodopa/carbidopa 250/25 mg) in Parkinson s disease: a single dose study]. Rev Med Chil. 2003 Jun;131(6):623-31. | ||||
| 5 | Vickers S, Stuart EK, Bianchine JR, Hucker HB, Jaffe ME, Rhodes RE, Vandenheuvel WJ: Metabolism of carbidopa (1-(-)-alpha-hydrazino-3,4-dihydroxy-alpha-methylhydrocinnamic acid monohydrate), an aromatic amino acid decarboxylase inhibitor, in the rat, rhesus monkey, and man. Drug Metab Dispos. 1974 Jan-Feb;2(1):9-22. | ||||
| 6 | Pharmacokinetics of levodopa/carbidopa microtablets versus levodopa/benserazide and levodopa/carbidopa in healthy volunteers. Clin Neuropharmacol. 2012 May-Jun;35(3):111-7. doi: 10.1097/WNF.0b013e31825645d1. | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Catechol-O-methyltransferase inhibitors in the management of Parkinson's disease. Semin Neurol. 2001;21(1):15-22. | ||||
| 9 | Molecular mechanisms controlling the rate and specificity of catechol O-methylation by human soluble catechol O-methyltransferase. Mol Pharmacol. 2001 Feb;59(2):393-402. doi: 10.1124/mol.59.2.393. | ||||
| 10 | Hardman JG, Limbird LE, Gilman AG, eds. "Goodman and Gilman's the Pharmacological Basis of Therapeutics. 10th ed." New York, NY: McGraw-Hill (2001):. | ||||
