Details of the Drug
General Information of Drug (ID: DMI0GDH)
| Drug Name |
Oxybuprocaine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Benoxil; Benoxinate; Butoxyaminobenzoyldiethylaminoethanol; Butoxyprocaine; Conjucain; Dorsacain; Novescine; Novesinol; Oxibuprocaina; Oxibuprocainum; Oxibuprokain; Oxybucaine; Oxybuprocainum; Oxyriprocaine; Prescaina; Benoxinate hydrochloride; Monofree oxybuprocaine; Oxybuprocaine HCL; Oxybuprocaine hydrochloride; S 749;Monofree oxybuprocaine (TN); Novesin (TN); Novesine (TN); Oxibuprocaina [INN-Spanish]; Oxybuprocaine (INN); Oxybuprocaine [INN:BAN]; Oxybuprocainum [INN-Latin]; Diethylaminoethyl-4-amino-3-butoxybenzoate; BENZOIC ACID, 4-AMINO-3-BUTOXY-, 2-(DIETHYLAMINO)ETHYL ESTER; Benzoic acid, 4-amino-3-butoxy-, 2-(diethylamino)ethyl ester (7CI,8CI,9CI); 2-(Diethylamino)ethyl 4-amino-3-butoxybenzoate; 2-(diethylamino)ethyl 4-amino-3-n-butoxybenzoate; 2-Diethylaminoethyl 4-amino-3-butoxybenzoate; 3-Butoxy-4-aminobenzoic acid 2-(diethylamino)ethyl ester; 3-Butoxy-4-aminobenzoic acid 2-diethylaminoethyl ester; 4-Amino-3-butoxy-2-(diethylamino)ethyl ester benzoic acid; 4-Amino-3-butoxy-benzoic acid 2-diethylamino-ethyl ester; 4-Amino-3-butoxybenzoic acid 2-(diethylamino)ethyl ester; 4-Amino-3-butoxybenzoicacid 2-diethylaminoethyl ester; 4-Amino-3-n-butoxy-benzoesaeure-diaethylaminoaethylester; 4-Amino-3-n-butoxy-benzoesaeure-diaethylaminoaethylester [German]
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anesthetics
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
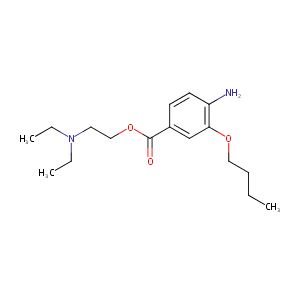 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 308.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 11 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
