Details of the Drug
General Information of Drug (ID: DMIUYFH)
| Drug Name |
Sulfapyridine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Adiplon; Coccoclase; Dagenan; Eubasin; Eubasinum; Haptocil; Piridazol; Plurazol; Pyriamid; Pyridazol; Relbapiridina; Ronin;Septipulmon; Solfapiridina; Streptosilpyridine; Sulfapiridina; Sulfapyridinum; Sulfidin; Sulfidine; Sulphapyridin; Sulphapyridine; Thioseptal; Trianon; Solfapiridina [DCIT]; M and B 693; A-499; ALBB-006215; M + B 693; M&B 693; M+B 693; Sulfapiridina [INN-Spanish]; Sulfapyridine (TN); Sulfapyridinum [INN-Latin]; AO-801/41077453; N(1)-Pyridylsulfanilamide; N(sup1)-Pyridylsulfanilamide; N-2-Pyridylsulfanilamide; N1-2-Pyridylsulfanilamide; Sulfapyridine (USP/INN); Sulfapyridine [USAN:INN:BAN]; N'-2-Pyridylsulfanilide; N(1)-2-Pyridylsulfanilamide; N(sup 1)-2-Pyridylsulfanilamide; N1-(Pyridin-2-yl)sulfanilamide; Sulfanilamide, N1-2-pyridyl-(8CI); 2-(4-Aminobenzenesulfonamido)pyridine; 2-(p-Aminobenzenesulphonamido)pyridine; 2-Sulfanilamidopyridin; 2-Sulfanilamidopyridin [German]; 2-Sulfanilamidopyridine; 2-Sulfanilyl aminopyridine; 2-Sulfanilylaminopyridine; 2-Sulfapyridine; 4-(2-Pyridinylsulfonyl)aniline; 4-AMINO-N-2-PYRIDINYLBENZENESULFONAMIDE; 4-Amino-N,2-pyridinylbenzenesulfonamide; 4-Amino-N-2-pyridinyl-benzenesulfonamide; 4-Amino-N-[2-pyridyl]benzene sulfonamide; 4-[(2-Pyridylamino)sulfonyl]aniline; 4-amino-N-(pyridin-2-yl)benzenesulfonamide; 4-amino-N-pyridin-2-yl-benzenesulfonamide; 4-amino-N-pyridin-2-ylbenzenesulfonamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Dermatologic Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
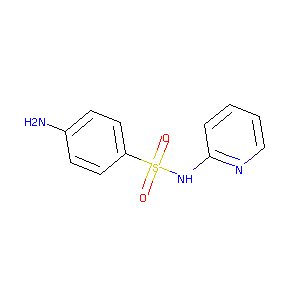 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 249.29 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
References
