Details of the Drug
General Information of Drug (ID: DMIZCOE)
| Drug Name |
Piperlongumine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Piplartine; 20069-09-4; Piperlongumin; UNII-SGD66V4SVJ; (E)-1-(3-(3,4,5-trimethoxyphenyl)acryloyl)-5,6-dihydropyridin-2(1H)-one; CHEBI:8241; SGD66V4SVJ; MFCD00075706; ST079382; 1-[(2E)-3-(3,4,5-trimethoxyphenyl)prop-2-enoyl]-1,2,5,6-tetrahydropyridin-2-one; 2(1H)-Pyridinone, 5,6-dihydro-1-[(2E)-1-oxo-3-(3,4,5-trimethoxyphenyl)-2-propen-1-yl]-; 5,6-Dihydro-1-[(2E)-1-oxo-3-(3,4,5-trimethoxyphenyl)-2-propen-1-yl]-2(1H)-pyridinone; BRD2293; Piplartin; BRD-2293; PPLGM; (E)-1-[3-(3,4,5-Trimethoxyphenyl)acryloyl]-5,6-dihydropyridin-2(1H)-one; 1-[(E)-3-(3,4,5-trimethoxyphenyl)prop-2-enoyl]-2,3-dihydropyridin-6-one; 5,6-Dihydro-1-(1-oxo-3-[3,4,5-trimethoxyphenyl]-trans-2-propenyl)-2[1H]-pyridinone; 5,6-Dihydro-1-[1-oxo-3-(3,4,5-trimethoxyphenyl)-trans-2-propenyl]-2(1H)-pyridinone; Piplartine;PPLGM; Prestwick_399; FERROUSFLUOBORATE; Prestwick2_000604; Prestwick3_000604; Piperlongumine; Piplartine; BSPBio_000508; MLS002153903; SCHEMBL173092; SPECTRUM1505135; BPBio1_000560; CHEMBL465843; SCHEMBL2465593; 1-[3-(3,4,5-Trimethoxy-phenyl)-acryloyl]-5,6-dihydro-1H-pyridin-2-one; ACon1_001541; CHEBI:92424; HMS1569J10; HMS2096J10; HMS2234K24; Piperlongumine, >=97% (HPLC); ZINC899053; BCP13030; EX-A2925; HY-N2329; 2659AH; BDBM50462013; NSC794671; s7551; AKOS024284776; CCG-214375; NSC-794671; 2(1H)-Pyridinone, 5,6-dihydro-1-(1-oxo-3-(3,4,5-trimethoxyphenyl)-2-propenyl)-, (E)-; NCGC00096028-01; NCGC00096028-02; NCGC00096028-03; NCGC00096028-04; NCGC00096028-14; AC-32683; AS-74140; BP-25401; LS-14579; SMR001233252; CS-0021113; P2361; A14124; C10166; SR-01000841248; A1-00162; J-012992; N-(3,4,5-Trimethoxycinnamoyl)-D3-piperidin-2-one; Q7197361; SR-01000841248-2; BRD-K24132293-001-05-3; BRD-K24132293-001-09-5; BRD-K24132293-001-16-0; 5,6-Dihydro-1-(3,4,5-trimethoxycinnamoyl)-2(1H)-pyridinone; 1-[(2E)-3-(3,4,5-Trimethoxyphenyl)-2-propenoyl]-5,6-dihydro-2(1H)-pyridinone #; 1-[(2E)-3-(3,4,5-trimethoxyphenyl)prop-2-enoyl]-1,5,6-trihydropyridin-2-one; 5,6-Dihydro-1-[1-oxo-3-(3,4,5-trimethoxyphenyl)-2-propenyl]-2(1H)-pyridinone, 9CI; Prop-2-en-1-one, 3-(3,4,5-trimethoxyphenyl)-1-(2,3-dihydropyridin-6(1H)-one-1-yl)-; (2E)-1-(1,2,5,6-Tetrahydro-2-oxopyridine-1-yl)-3-(3,4,5-trimethoxyphenyl)-2-propene-1-one
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
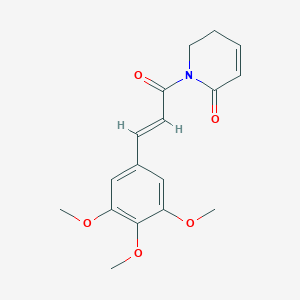 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 317.34 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Experimental Cancer Drug Sensitivity Information
References
| 1 | Piperlongumine, a potent anticancer phytotherapeutic: Perspectives on contemporary status and future possibilities as an anticancer agent. Pharmacol Res. 2020 Jun;156:104772. | ||||
|---|---|---|---|---|---|
| 2 | Activity-based protein profiling reveals GSTO1 as the covalent target of piperlongumine and a promising target for combination therapy for cancer. Chem Commun (Camb). 2019 Apr 9;55(30):4407-4410. | ||||
| 3 | Piperlongumine induces ROS mediated apoptosis by transcriptional regulation of SMAD4/P21/P53 genes and synergizes with doxorubicin in osteosarcoma cells. Chem Biol Interact. 2022 Feb 25;354:109832. doi: 10.1016/j.cbi.2022.109832. Epub 2022 Jan 24. | ||||
| 4 | Piperlongumine analogue L50377 induces pyroptosis via ROS mediated NF-B suppression in non-small-cell lung cancer. Chem Biol Interact. 2019 Nov 1;313:108820. doi: 10.1016/j.cbi.2019.108820. Epub 2019 Sep 10. | ||||
| 5 | Piperlongumine decreases cell proliferation and the expression of cell cycle-associated proteins by inhibiting Akt pathway in human lung cancer cells. Food Chem Toxicol. 2018 Jan;111:9-18. doi: 10.1016/j.fct.2017.10.058. Epub 2017 Nov 7. | ||||
| 6 | Piperlongumine is a novel nuclear export inhibitor with potent anticancer activity. Chem Biol Interact. 2015 Jul 25;237:66-72. doi: 10.1016/j.cbi.2015.05.016. Epub 2015 May 28. | ||||
