Details of the Drug
General Information of Drug (ID: DMJPGUA)
| Drug Name |
MANIDIPINE
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
manidipine; 89226-50-6; Franidipine; 120092-68-4; Manidipine 6300; 3-(2-(4-Benzhydrylpiperazin-1-yl)ethyl) 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate; Iperten; Artedil; Manidipine [INN]; Manidipine (Manyper); C35H38N4O6; CHEMBL312176; Manidipine (INN); 2-(4-Diphenylmethyl-1-piperazinyl)ethyl methyl-1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate; 2-[4-(diphenylmethyl)piperazin-1-yl]ethyl methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridin
|
|||||
| Affected Organisms |
Humans and other mammals
|
|||||
| ATC Code | ||||||
| Drug Type |
Small molecular drug
|
|||||
| Structure |
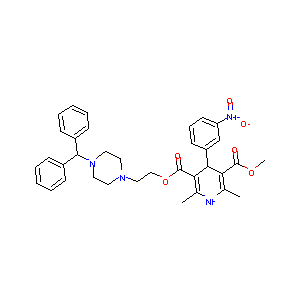 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 610.7 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 5.6 | |||||
| Rotatable Bond Count (rotbonds) | 11 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
