Details of the Drug
General Information of Drug (ID: DMJWT3X)
| Drug Name |
HONOKIOL
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Honokiol; 35354-74-6; 5,3'-Diallyl-2,4'-dihydroxybiphenyl; NSC 293100; Honokiol,(S); UNII-11513CCO0N; 3,5'-Diallyl-4,2'-dihydroxybiphenyl; 3',5-Diallylbiphenyl-2,4'-diol; CPD000387107; CHEMBL16901; CHEBI:5759; 4-allyl-2-(3-allyl-4-hydroxy-phenyl)phenol; 3',5-diallyl-[1,1'-biphenyl]-2,4'-diol; 11513CCO0N; 3',5-diallyl-2,4'-biphenyldiol; AK-25837; 5,3& -Diallyl-2,4& Q-100425; [1,1'-Biphenyl]-2,4'-diol, 3',5-di-2-propenyl-; SMR000387107; 3',5-di(prop-2-en-1-yl)biphenyl-2,4'-diol; houpa
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
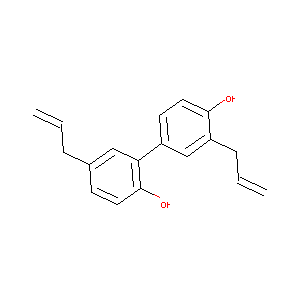 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 266.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
