Details of the Drug
General Information of Drug (ID: DMLT4JA)
| Drug Name |
UDP-glucose
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Uridine-5'-diphosphate-glucose; URIDINE DIPHOSPHATE GLUCOSE; UDPG; UDP-alpha-D-Glucose; 133-89-1; UNII-V50K1D7P4Y; URIDINE-5'-DIPHOSPHATE-GLUCOSE; 5'-Diphosphoglucose; UDP-D-glucose; GLUCOSE-URIDINE-C1,5'-DIPHOSPHATE; UDPglucose; V50K1D7P4Y; CHEBI:46229; URIDINE-5'-DIPHOSPHOGLUCOSE; UDP-Glc; Uridine diphosphoglucose; Uridine 5'-diphosphoglucose; co-waldenase; URIDINE-5'-MONOPHOSPHATE GLUCOPYRANOSYL-MONOPHOSPHATE ESTER; co-galactoisomerase; [[(2R,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]; uridine diphosphate glucose; Glucose-Uridine-C1,5'-Diphosphate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
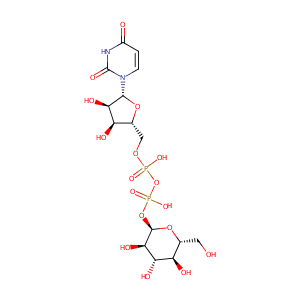 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 566.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -6.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 17 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
