| Synonyms |
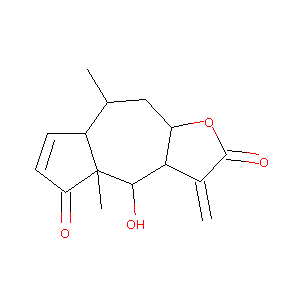
HELENALIN; 6754-13-8; PF 56; Helenalin A; HSDB 3490; NSC85236; UNII-4GUY9L896T; NSC 85236; BRN 0028081; MLS000728512; CHEBI:5635; 4GUY9L896T; 6alpha,8beta-Dihydroxy-4-oxoambrosa-2,11(13)-dien-12-oic acid 12,8-lactone; SMR000445626; Ambrosa-2,11(13)-dien-12-oic acid, 6alpha,8beta-dihydroxy-4-oxo-, 12,8-lactone; (3aR,5R,5aR,8aR,9S,9aS)-9-hydroxy-5,8a-dimethyl-1-methylene-3a,4,5,5a,9,9a-hexahydroazuleno[6,7-b]furan-2,8-dione; Azuleno(6,5-b)furan-2,5-dione, 3,3a,4,4a,7a,8,9,9a-octahydro-4-hydroxy-4a,8-dimethyl-3-methylene-, (3
|
| Chemical Identifiers |
- Formula
- C15H18O4
- IUPAC Name
(3aR,5R,5aR,8aR,9S,9aS)-9-hydroxy-5,8a-dimethyl-1-methylidene-3a,4,5,5a,9,9a-hexahydroazuleno[6,7-b]furan-2,8-dione - Canonical SMILES
-
C[C@@H]1C[C@@H]2[C@H]([C@@H]([C@]3([C@H]1C=CC3=O)C)O)C(=C)C(=O)O2
- InChI
-
InChI=1S/C15H18O4/c1-7-6-10-12(8(2)14(18)19-10)13(17)15(3)9(7)4-5-11(15)16/h4-5,7,9-10,12-13,17H,2,6H2,1,3H3/t7-,9+,10-,12-,13+,15+/m1/s1
- InChIKey
-
ZVLOPMNVFLSSAA-XEPQRQSNSA-N
|