Details of the Drug
General Information of Drug (ID: DMP7X6Q)
| Drug Name |
Rotigotine
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Rotigotine; Rotigotine CDS; Rotigotine CDS Patch; SPM 962; SPM-962; (-)-(S)-5,6,7,8-Tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)-1-naphthol; Leganto; N 0923; Neupro; (6S)-6-(Propyl(2-(2-thienyl)ethyl)amino)-5,6,7,8-tetrahydro-1-naphthalenol; (6S)-6-[propyl(2-thiophen-2-ylethyl)amino]-5,6,7,8-tetrahydronaphthalen-1-ol; (S)-6-(propyl(2-(thiophen-2-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol; 87T4T8BO2E; 99755-59-6; C19H25NOS; CHEMBL1303; DSSTox_CID_26772; DSSTox_RID_81893; NCGC00168748-01; UNII-87T4T8BO2E
|
|||||
| Structure |
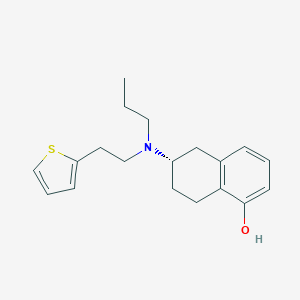 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 315.5 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 4.9 | |||||
| Rotatable Bond Count (rotbonds) | 6 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
