| Synonyms |
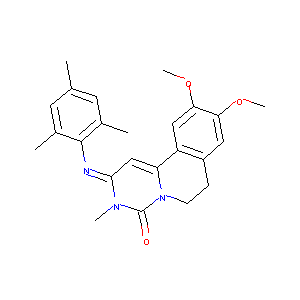
trequinsin; Trequinsin [INN]; Trequinsinum [INN-Latin]; Trequinsine [INN-French]; 79855-88-2; UNII-739I2958C1; CHEMBL285913; (2e)-2-(mesitylimino)-9,10-dimethoxy-3-methyl-2,3,6,7-tetrahydro-4h-pyrimido[6,1-a]isoquinolin-4-one; 739I2958C1; Trequinsinum; Trequinsine; C24H27N3O3; 2,3,6,7-Tetrahydro-2-(mesitylimino)-9,10-dimethoxy-3-methyl-4H-pyrimido(6,1-a)isoquinolin-4-one; AC1L1KKE; AC1Q6LSV; Lopac-T-2057; Lopac0_001165; KBioGR_000072; KBioSS_000072; BSPBio_001352; SCHEMBL2777196; SCHEMBL2396911; CHEMBL588593; CHEMBL1411581
|
| Chemical Identifiers |
- Formula
- C24H27N3O3
- IUPAC Name
9,10-dimethoxy-3-methyl-2-(2,4,6-trimethylphenyl)imino-6,7-dihydropyrimido[6,1-a]isoquinolin-4-one - Canonical SMILES
-
CC1=CC(=C(C(=C1)C)N=C2C=C3C4=CC(=C(C=C4CCN3C(=O)N2C)OC)OC)C
- InChI
-
InChI=1S/C24H27N3O3/c1-14-9-15(2)23(16(3)10-14)25-22-13-19-18-12-21(30-6)20(29-5)11-17(18)7-8-27(19)24(28)26(22)4/h9-13H,7-8H2,1-6H3
- InChIKey
-
MCMSJVMUSBZUCN-UHFFFAOYSA-N
|