Details of the Drug
General Information of Drug (ID: DMRJKIV)
| Drug Name |
Caffeic acid phenethyl ester
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Caffeic acid phenethyl ester; Phenethyl caffeate; 104594-70-9; CAPE; phenethyl 3-(3,4-dihydroxyphenyl)acrylate; Capeee; Phenylethyl caffeate; 115610-29-2; caffeic acid phenylethyl ester; UNII-G960R9S5SK; 2-phenylethyl caffeate; PHENETHYL CAFFEATE (CAPE); Caffeic acid-phenethyl ester; CHEBI:8062; G960R9S5SK; CHEMBL319244; 100981-80-4; Caffeic acid 2-phenylethyl ester; SWUARLUWKZWEBQ-VQHVLOKHSA-N; 2-phenylethyl 3-(3,4-dihydroxyphenyl)-2-propenoate; Caffeic Acid Phenethyl Ester, Synthetic; 2-Phenylethyl (2e)-3-(3,4-Dihydroxyphenyl
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
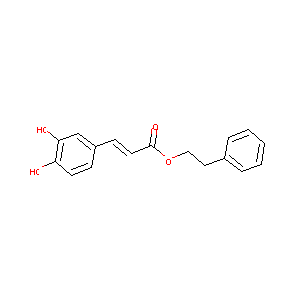 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 284.31 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
