| Drug Name |
ACMC-1AKLT
|
| Synonyms |
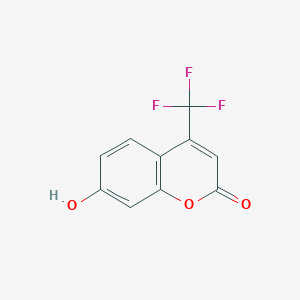
Maybridge1_006885; PubChem8688; SCHEMBL307508; ZINC57916; 2H-1-Benzopyran-2-one, 7-hydroxy-4-(trifluoromethyl)-; 4-(Trifluoromethyl)umbelliferone; 4-(trifluoromethyl)umbeilliferone; 575-03-1; 7,4-Hfc; 7-Hydroxy-4-(trifluoromethyl)-2H-chromen-2-one; 7-Hydroxy-4-(trifluoromethyl)coumarin; 7-Hydroxy-4-trifluoromethyl-chromen-2-one; 7-Hydroxy-4-trifluoromethylcoumarin; 7-hydroxy-4-(trifluoromethyl)chromen-2-one; AC1NT9P6; AC1Q795W; CCKWMCUOHJAVOL-UHFFFAOYSA-N; CHEMBL104679; CTK5A6979; HMS561A21; MFCD00037578; TFMU
|
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
230.14 |
|
| Logarithm of the Partition Coefficient (xlogp) |
2.4 |
| Rotatable Bond Count (rotbonds) |
0 |
| Hydrogen Bond Donor Count (hbonddonor) |
1 |
| Hydrogen Bond Acceptor Count (hbondacc) |
6 |
| Chemical Identifiers |
- Formula
- C10H5F3O3
- IUPAC Name
7-hydroxy-4-(trifluoromethyl)chromen-2-one - Canonical SMILES
-
C1=CC2=C(C=C1O)OC(=O)C=C2C(F)(F)F
- InChI
-
CCKWMCUOHJAVOL-UHFFFAOYSA-N
- InChIKey
-
1S/C10H5F3O3/c11-10(12,13)7-4-9(15)16-8-3-5(14)1-2-6(7)8/h1-4,14H
|
| Cross-matching ID |
- PubChem CID
- 5375667
- CAS Number
-
- INTEDE ID
- DR1937
|
|
|
|
|
|
|
|



