Details of the Drug
General Information of Drug (ID: DMTX2BH)
| Drug Name |
Cefonicid
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cefonicido; Cefonicidum; Monocef; Praticef; Cefonicid Monosodium Salt; Sodium Cefonicid; Cefonicid (INN); Cefonicid [INN:BAN]; Cefonicido [INN-Spanish]; Cefonicidum [INN-Latin]; Monocef (TN); SK&F-75073; SKF-75073-2; (6R,7R)-7-[(2-hydroxy-2-phenylacetyl)amino]-8-oxo-3-[[1-(sulfomethyl)tetrazol-5-yl]sulfanylmethyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2R)-2-hydroxy-2-phenylacetyl]amino]-8-oxo-3-[[1-(sulfomethyl)tetrazol-5-yl]sulfanylmethyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2R)-2-hydroxy-2-phenylacetyl]amino}-8-oxo-3-({[1-(sulfomethyl)-1H-tetrazol-5-yl]sulfanyl}methyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2R)-2-hydroxy-2-phenylacetyl]amino}-8-oxo-3-({[1-(sulfomethyl)-1H-tetrazol-5-yl]thio}methyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (7R)-7-(2-Hydroxy-2-phenylacetylamino)-6-oxo-3-{[1-(sulfomethyl)(1,2,3,4-tetraazol-5-ylthio)]methyl}-2H,7H,7aH-azetidino[2,1-b]1,3-thiazine-4-carboxylic acid; 6beta-[(2R)-2-hydroxy-2-phenylacetamido]-3-({[1-(sulfomethyl)-1H-tetrazol-5-yl]sulfanyl}methyl)ceph-3-em-4-carboxylic acid; 7-[(2-hydroxy-2-phenylacetyl)amino]-8-oxo-3-[[1-(sulfomethyl)tetrazol-5-yl]sulfanylmethyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteria
|
||||||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
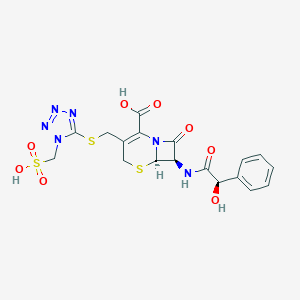 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 542.6 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.9 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 13 | ||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Cefonicid (Comorbidity)
|
|||||||||||||||||||||||||||||
References
