Details of the Drug
General Information of Drug (ID: DMVP4YK)
| Drug Name |
CB1954
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Tretazicar; CB 1954; 21919-05-1; CB1954; 5-(1-Aziridinyl)-2,4-dinitrobenzamide; 5-Aziridino-2,4-dinitrobenzamide; CB-1954; 5-(AZIRIDIN-1-YL)-2,4-DINITROBENZAMIDE; 5-Aziridinyl-2,4-dinitrobenzamide; Tretazicar [INN]; 2,4-Dinitroethyleneiminobenzamide; 2,4-Dinitro-5-ethyleneiminobenzamide; CHEMBL23330; CCRIS 1631; UNII-7865D5D01M; NSC 115829; 7865D5D01M; BRN 5825582; Benzamide,5-(1-aziridinyl)-2,4-dinitro-; Benzamide, 5-(1-aziridinyl)-2,4-dinitro-; CB1; C9H8N4O5; SMR000326847; aziridine dinitrobenzamide; CB1954(Tretazicar); AC1Q1YAR
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
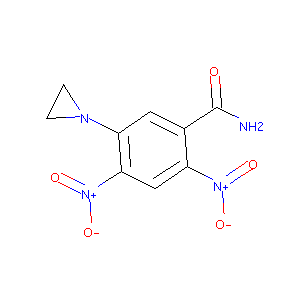 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 252.18 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
References
