Details of the Drug
General Information of Drug (ID: DMW3QGJ)
| Drug Name |
LM-94
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Bilcolic; Bilicante; Hymecromone; Cantabilin; Cantabiline; Cholestil; Cholonerton; Coumarin 4; Crodimon; Cumarote-C; Eurogale; Himecromona; Hymecromon; Hymecromonum; Imecromone; Imecromone [DCIT]; Medilla; Mendiaxon; Omega 127; Pilot 447; Resocyanine; beta-Methylumbelliferone; 2H-1-Benzopyran-2-one, 7-hydroxy-4-methyl-; 4-MU; 4-Methyl-7-hydroxycoumarin; 4-Methylumbelliferon; 4-methylumbelliferone; 7-HYDROXY-4-METHYLCOUMARIN; 7-Hydroxy-4-methyl-2-oxo-2H-1-benzopyran; 7-Hydroxy-4-methyl-2H-chromen-2-one; 90-33-5; LM 94; NSC 19026; NSC 9408
|
|||||
| ATC Code | ||||||
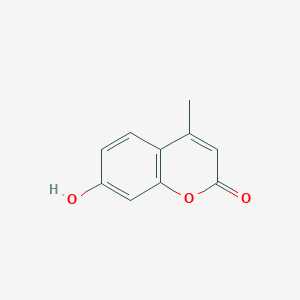
| Structure |
 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 176.17 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 1.9 | |||||
| Rotatable Bond Count (rotbonds) | 0 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
