Details of the Drug
General Information of Drug (ID: DMWIJTN)
| Drug Name |
Voclosporin
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Luveniq; ISAtx-247; ISA-247; UNII-2PN063X6B1; ISATX247; 515814-01-4; ISA247; 2PN063X6B1; ISA 247; trans-ISA-247; LX211; Voclosporin (USAN/INN); Voclosporin [USAN:INN]; Voclera; 3odi; LX-211; LX-214; ISA247, Luveniq; AC1OCFHS; R-1524; SCHEMBL12632344; CHEBI:135957; (E)-ISA-247; DB11693; 515814-00-3; HY-106638; CS-0026210; D09033; Cyclosporin A, 6-((2S,3R,4R)-3-hydroxy-4-methyl-2-(methylamino)-6,8-nonadienoic acid)-; 368455-04-3; Luveniq; Voclosporin [USAN]; R 1524; Trans-ISA 247; Trans-ISA-247; TrkA-IgG
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
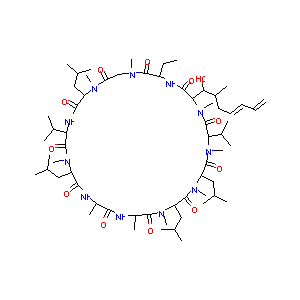 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 1214.6 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 7.9 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 16 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 12 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Lupus nephritis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 4A40.0Y | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
