Details of the Drug
General Information of Drug (ID: DMY29Q8)
| Drug Name |
Calyculin-A
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
calyculin A; C05370; 101932-71-2; [(3R,5R,7R,8S,9S)-2-[(1S,3S,4S,5R,6R,7E,9E,11E,13Z)-14-cyano-3,5-dihydroxy-1-methoxy-4,6,8,9,13-pentamethyl-tetradeca-7,9,11,13-tetraenyl]-9-[(E)-3-[2-[(1S)-3-[[(2S,3S,4S)-4-(dimethylamino)-2,3-dihydroxy-5-methoxy-pentanoyl]amino]-1-methyl-propyl]oxazol-4-yl]allyl]-7-hydroxy-4,4,8-trimethyl-1,10-dioxaspiro[4.5]decan-3-yl] dihydrogen phosphate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
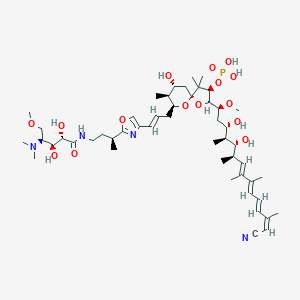 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 1009.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 26 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 18 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
References
