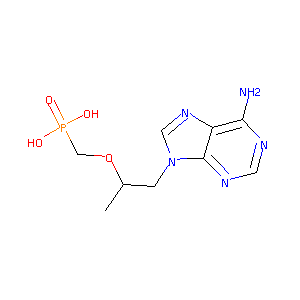
| 1 |
ClinicalTrials.gov (NCT03917420) Quantification of Estradiol's Impact on Nucleotides in Cellular Populations of the Lower GI Tract
|
| 2 |
Tenofovir FDA Label
|
| 3 |
Anti-hepatitis B virus activity in vitro of combinations of tenofovir with nucleoside/nucleotide analogues. Antivir Chem Chemother. 2009;19(4):165-76.
|
| 4 |
Nat Rev Drug Discov. 2013 Feb;12(2):87-90.
|
| 5 |
Clinical pipeline report, company report or official report of Gilead (2011).
|
| 6 |
Antiviral drugs in current clinical use. J Clin Virol. 2004 Jun;30(2):115-33.
|
| 7 |
KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01665)
|
| 8 |
KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01913)
|
| 9 |
Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007 Feb;71(2):619-27.
|
| 10 |
Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids. 2001 Apr-Jul;20(4-7):641-8.
|
| 11 |
Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir Ther. 2014;19(7):687-92.
|
| 12 |
Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J Infect Dis. 2011 Jul 1;204(1):145-53.
|
| 13 |
Tenofovir Disoproxil Fumarate Is a New Substrate of ATP-Binding Cassette Subfamily C Member 11. Antimicrob Agents Chemother. 2017 Mar 24;61(4). pii: e01725-16.
|
| 14 |
Emtricitabine is a substrate of MATE1 but not of OCT1, OCT2, P-gp, BCRP or MRP2 transporters. Xenobiotica. 2017 Jan;47(1):77-85.
|
| 15 |
Chronic Nucleoside Reverse Transcriptase Inhibitors Disrupt Mitochondrial Homeostasis and Promote Premature Endothelial Senescence. Toxicol Sci. 2019 Dec 1;172(2):445-456. doi: 10.1093/toxsci/kfz203.
|
| 16 |
Binding of anti-HIV drugs to human serum albumin. IUBMB Life. 2004 Oct;56(10):609-14. doi: 10.1080/15216540400016286.
|
| 17 |
Nonenantioselectivity property of human deoxycytidine kinase explained by structures of the enzyme in complex with L- and D-nucleosides. J Med Chem. 2007 Jun 28;50(13):3004-14. doi: 10.1021/jm0700215. Epub 2007 May 27.
|
| 18 |
ClinicalTrials.gov (NCT03360682) Clinical Trial to Evaluate the Efficacy, Pharmacokinetics (PK) Interactions and Safety of Dolutegravir Plus 2 Nucleoside Reverse Transcriptase Inhibitors (NRTIs) in HIV-1-Infected Solid Organ Transplant Patients
|
| 19 |
ClinicalTrials.gov (NCT01335620) The Safety, Pharmacokinetic Profile and Efficacy of Raltegravir in HIV-infected Patients at Least 60 Years of Age
|
| 20 |
ClinicalTrials.gov (NCT01803074) Study to Evaluate a HIV Drug for the Treatment of HIV Infection
|
| 21 |
ClinicalTrials.gov (NCT02475915) Efficacy of VHM After Treatment Interruption in Subjects Initiating ART During Acute HIV Infection
|
|
|
|
|
|
|