| 1 |
ClinicalTrials.gov (NCT00930865) Study to Evaluate the Potential Pharmacokinetic Interaction and Pharmacodynamic Effects on Renal Parameters of Bumetanide (1mg) and Dapagliflozin (10 mg) When Co-administered in Healthy Subjects
|
| 2 |
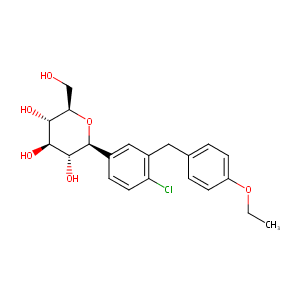
Dapagliflozin FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4594).
|
| 4 |
Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19)
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4837).
|
| 6 |
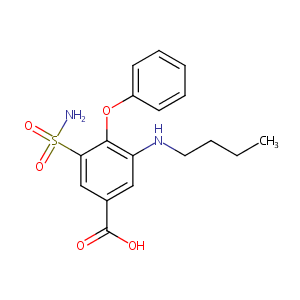
Bumetanide FDA Label
|
| 7 |
2014 FDA drug approvals. Nat Rev Drug Discov. 2015 Feb;14(2):77-81.
|
| 8 |
Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet. 2014 Jan;53(1):17-27.
|
| 9 |
Discovery of novel N--D-xylosylindole derivatives as sodium-dependent glucose cotransporter 2 (SGLT2) inhibitors for the management of hyperglycemia in diabetes. J Med Chem. 2011 Jan 13;54(1):166-78. doi: 10.1021/jm101072y. Epub 2010 Dec 3.
|
| 10 |
Nerve Terminal GABAA Receptors Activate Ca2+/Calmodulin-dependent Signaling to Inhibit Voltage-gated Ca2+ Influx and Glutamate Release. J Biol Chem. 2009 Mar 27;284(13):8726-37.
|
| 11 |
Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J Pharm Pharmacol. 2005 May;57(5):573-8.
|
| 12 |
Interactions of human organic anion transporters with diuretics. J Pharmacol Exp Ther. 2004 Mar;308(3):1021-9.
|
| 13 |
Genetic variation in the renal sodium transporters NKCC2, NCC, and ENaC in relation to the effects of loop diuretic drugs. Clin Pharmacol Ther. 2007 Sep;82(3):300-9.
|
| 14 |
Quercetin, Morin, Luteolin, and Phloretin Are Dietary Flavonoid Inhibitors of Monocarboxylate Transporter 6. Mol Pharm. 2017 Sep 5;14(9):2930-2936.
|
| 15 |
Dosage dependent hormonal counter regulation to combination therapy in patients with left ventricular dysfunction. J Clin Pharm Ther. 2006 Apr;31(2):139-47. doi: 10.1111/j.1365-2710.2006.00606.x.
|
| 16 |
Effects of a new cystic fibrosis transmembrane conductance regulator inhibitor on Cl- conductance in human sweat ducts. Exp Physiol. 2004 Jul;89(4):417-25. doi: 10.1113/expphysiol.2003.027003. Epub 2004 May 6.
|
| 17 |
Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3.
|
| 18 |
Azosemide is more potent than bumetanide and various other loop diuretics to inhibit the sodium-potassium-chloride-cotransporter human variants hNKCC1A and hNKCC1B. Sci Rep. 2018 Jun 29;8(1):9877. doi: 10.1038/s41598-018-27995-w.
|
| 19 |
Functional analysis of human sodium-phosphate transporter 4 (NPT4/SLC17A3) polymorphisms. J Pharmacol Sci. 2011;115(2):249-53. doi: 10.1254/jphs.10228sc. Epub 2011 Jan 26.
|
| 20 |
Multichannel liquid chromatography-tandem mass spectrometry cocktail method for comprehensive substrate characterization of multidrug resistance-associated protein 4 transporter. Pharm Res. 2007 Dec;24(12):2281-96.
|
|
|
|
|
|
|