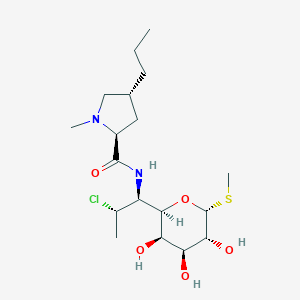
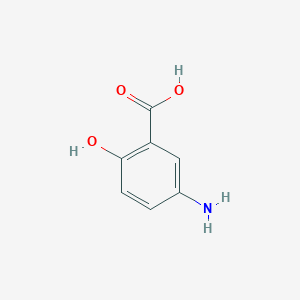
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 3 |
Clindamycin FDA Label
|
| 4 |
Colonic diverticular disease. Nat Rev Dis Primers. 2020 Mar 26;6(1):20.
|
| 5 |
Optimized Management of Ulcerative Proctitis: When and How to Use Mesalazine Suppository. Digestion. 2018;97(1):59-63.
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4655).
|
| 7 |
Efficacy of Oral, Topical, or Combined Oral and Topical 5-Aminosalicylates, in Ulcerative Colitis: Systematic Review and Network Meta-analysis. J Crohns Colitis. 2021 Jul 5;15(7):1184-1196.
|
| 8 |
Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature. 2001 Oct 25;413(6858):814-21.
|
| 9 |
In vitro metabolism of clindamycin in human liver and intestinal microsomes. Drug Metab Dispos. 2003 Jul;31(7):878-87.
|
| 10 |
Comparison of antimicrobial susceptibility, beta-lactamase production, plasmid analysis and serum bactericidal activity in Edwardsiella tarda, E. ictaluri and E. hoshinae. J Med Microbiol. 1993 Oct;39(4):273-81.
|
| 11 |
A comprehensive in vitro and in silico analysis of antibiotics that activate pregnane X receptor and induce CYP3A4 in liver and intestine. Drug Metab Dispos. 2008 Aug;36(8):1689-97.
|
| 12 |
Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3.
|
| 13 |
Chloramphenicol causes mitochondrial stress, decreases ATP biosynthesis, induces matrix metalloproteinase-13 expression, and solid-tumor cell invasion. Toxicol Sci. 2010 Jul;116(1):140-50. doi: 10.1093/toxsci/kfq085. Epub 2010 Mar 25.
|
| 14 |
How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6.
|
| 15 |
Transport studies with 5-aminosalicylate. Eur J Clin Pharmacol. 2006 Oct;62(10):871-5.
|
| 16 |
Role of organic anion-transporting polypeptides for cellular mesalazine (5-aminosalicylic acid) uptake. Drug Metab Dispos. 2011 Jun;39(6):1097-102.
|
| 17 |
NAT1 genotypes do not predict response to mesalamine in patients with ulcerative colitis. Z Gastroenterol. 2008 Mar;46(3):259-65.
|
|
|
|
|
|
|