| 1 |
ClinicalTrials.gov (NCT02551536) Montelukast and Fexofenadine Versus Montelukast and Levocetrizine Combination in Allergic Rhinitis
|
| 2 |
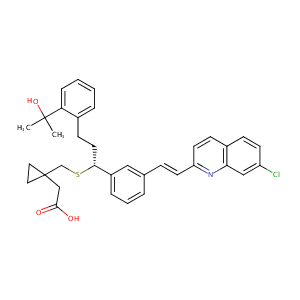
Montelukast FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3340).
|
| 4 |
The COvid-19 Symptom MOntelukast Trial (COSMO)
|
| 5 |
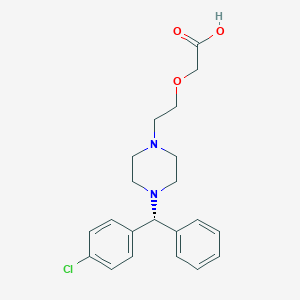
Levocetirizine FDA Label
|
| 6 |
As a potential treatment of COVID-19: Montelukast. Med Hypotheses. 2020 May 11;142:109828.
|
| 7 |
Protective potential of montelukast against hepatic ischemia/reperfusion injury in rats. J Surg Res. 2010 Mar;159(1):588-94.
|
| 8 |
Effect of citrus juice and SLCO2B1 genotype on the pharmacokinetics of montelukast. J Clin Pharmacol. 2011 May;51(5):751-60.
|
| 9 |
Effects of polymorphisms of the SLCO2B1 transporter gene on the pharmacokinetics of montelukast in humans. J Clin Pharmacol. 2013 Nov;53(11):1186-93.
|
| 10 |
Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675.
|
| 11 |
Determinants of cytochrome P450 2C8 substrate binding: structures of complexes with montelukast, troglitazone, felodipine, and 9-cis-retinoic acid. J Biol Chem. 2008 Jun 20;283(25):17227-37.
|
| 12 |
In vitro metabolism of montelukast by cytochrome P450s and UDP-glucuronosyltransferases. Drug Metab Dispos. 2015 Dec;43(12):1905-16.
|
| 13 |
Cellular Uptake of Levocetirizine by Organic Anion Transporter 4. J Pharm Sci. 2017 Sep;106(9):2895-2898.
|
|
|
|
|
|
|