| 1 |
ClinicalTrials.gov (NCT03209401) Niraparib Plus Carboplatin in Patients With Homologous Recombination Deficient Advanced Solid Tumor Malignancies
|
| 2 |
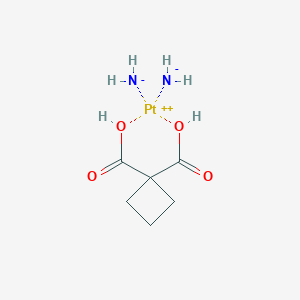
Carboplatin FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7624).
|
| 4 |
ClinicalTrials.gov (NCT03602859) A Phase 3 Comparison of Platinum-based Therapy With TSR-042 and Niraparib Versus Standard of Care (SOC) Platinum-based Therapy as First-line Treatment of Stage III or IV Nonmucinous Epithelial Ovarian Cancer (FIRST). U.S. National Institutes of Health.
|
| 5 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 6 |
Inhibition of carboplatin-induced DNA interstrand cross-link repair by gemcitabine in patients receiving these drugs for platinum-resistant ovarian cancer.Clin Cancer Res.2010 Oct 1;16(19):4899-905.
|
| 7 |
Overcoming platinum drug resistance with copper-lowering agents. Anticancer Res. 2013 Oct;33(10):4157-61.
|
| 8 |
PharmGKB: A worldwide resource for pharmacogenomic information. Wiley Interdiscip Rev Syst Biol Med. 2018 Jul;10(4):e1417. (ID: PA150642262)
|
| 9 |
Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41.
|
| 10 |
Discovery of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): a novel oral poly(ADP-ribose)polymerase (PARP) inhibitor efficacious in BRCA-1 and -2 mutant tumors. J Med Chem. 2009 Nov 26;52(22):7170-85.
|
| 11 |
Summary of FDA-approved anticancer cytotoxic drugs at May 2019.
|
| 12 |
Autophagy up-regulated by MEK/ERK promotes the repair of DNA damage caused by aflatoxin B1. Toxicol Mech Methods. 2022 Feb;32(2):87-96. doi: 10.1080/15376516.2021.1968985. Epub 2021 Aug 26.
|
|
|
|
|
|
|