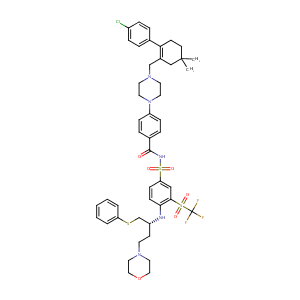
| 1 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
| 2 |
ClinicalTrials.gov (NCT04472598) Study of Oral Navitoclax Tablet In Combination With Oral Ruxolitinib Tablet When Compared With Oral Ruxolitinib Tablet To Assess Change In Spleen Volume In Adult Participants With Myelofibrosis (TRANSFORM-1). U.S. National Institutes of Health.
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8319).
|
| 4 |
Small molecules, big targets: drug discovery faces the protein-protein interaction challenge.Nat Rev Drug Discov. 2016 Aug;15(8):533-50.
|
| 5 |
Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009941)
|
| 6 |
Clinical pipeline report, company report or official report of Roche (2009).
|
| 7 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 8 |
The B-cell lymphoma 2 (BCL2)-inhibitors, ABT-737 and ABT-263, are substrates for P-glycoprotein. Biochem Biophys Res Commun. 2011 May 6;408(2):344-9.
|
| 9 |
Effect of rifampin on the pharmacokinetics, safety and tolerability of navitoclax (ABT-263), a dual inhibitor of Bcl-2 and Bcl-XL , in patients with cancer. J Clin Pharm Ther. 2014 Dec;39(6):680-4.
|
| 10 |
Human breast cancer cells display different sensitivities to ABT-263 based on the level of survivin. Toxicol In Vitro. 2018 Feb;46:229-236. doi: 10.1016/j.tiv.2017.09.023. Epub 2017 Sep 23.
|
| 11 |
BCL2/BCL-X(L) inhibition induces apoptosis, disrupts cellular calcium homeostasis, and prevents platelet activation. Blood. 2011 Jun 30;117(26):7145-54. doi: 10.1182/blood-2011-03-344812. Epub 2011 May 11.
|
| 12 |
Inhibition of rat brain monoamine oxidase activities by psoralen and isopsoralen: implications for the treatment of affective disorders. Pharmacol Toxicol. 2001 Feb;88(2):75-80.
|
| 13 |
Emerging therapies for multiple myeloma. Expert Opin Emerg Drugs. 2009 Mar;14(1):99-127.
|
| 14 |
Pharmacological targeting of NF-kappaB potentiates the effect of the topoisomerase inhibitor CPT-11 on colon cancer cells. Br J Cancer. 2008 Jan 29;98(2):335-44. doi: 10.1038/sj.bjc.6604082. Epub 2008 Jan 8.
|
|
|
|
|
|
|