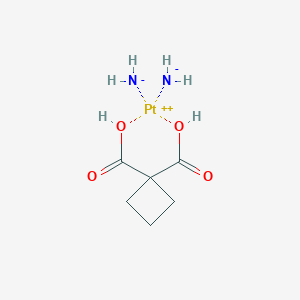
| Molecular Interaction Atlas (MIA) |
|
| Indication(s) of Carboplatin |
| Disease Entry |
ICD 11 |
Status |
REF |
| Adenocarcinoma |
2D40
|
Approved |
[2] |
| Carcinoma |
2A00-2F9Z
|
Approved |
[2] |
| Cholangiocarcinoma |
2C12.10
|
Approved |
[2] |
| Cutaneous melanoma |
2C30
|
Approved |
[2] |
| Epithelial ovarian cancer |
2B5D
|
Approved |
[2] |
| Fallopian tube neoplasm |
N.A.
|
Approved |
[2] |
| Gastroesophageal junction adenocarcinoma |
2B71
|
Approved |
[2] |
| Lung adenocarcinoma |
N.A.
|
Approved |
[2] |
| Lung cancer |
2C25.0
|
Approved |
[2] |
| Non-small-cell lung cancer |
2C25.Y
|
Approved |
[2] |
| Ovarian cancer |
2C73
|
Approved |
[3] |
| Ovarian disorder |
N.A.
|
Approved |
[2] |
| Ovarian neoplasm |
N.A.
|
Approved |
[2] |
| Ovarian serous cystadenocarcinoma |
N.A.
|
Approved |
[2] |
| Small-cell lung cancer |
2C25.Y
|
Approved |
[2] |
| Lung large cell carcinoma |
N.A.
|
Investigative |
[2] |
| Neuroblastoma |
2D11.2
|
Investigative |
[2] |
|
|
|
Carboplatin Interacts with 1 DTT Molecule(s)
| DTT Name |
DTT ID |
UniProt ID |
Mode of Action |
REF |
|
Human Deoxyribonucleic acid (hDNA)
|
TTUTN1I
|
NOUNIPROTAC
|
Modulator
|
[6] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
|
|
Carboplatin Interacts with 1 DTP Molecule(s)
| DTP Name |
DTP ID |
UniProt ID |
Mode of Action |
REF |
|
High affinity copper uptake protein 1 (SLC31A1)
|
DTP8L4F
|
COPT1_HUMAN
|
Substrate
|
[7] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
|
|
Carboplatin Interacts with 7 DME Molecule(s)
| DME Name |
DME ID |
UniProt ID |
Mode of Action |
REF |
|
Glutathione S-transferase pi (GSTP1)
|
DEK6079
|
GSTP1_HUMAN
|
Metabolism
|
[8] |
|
Glutathione S-transferase theta-1 (GSTT1)
|
DE3PKUG
|
GSTT1_HUMAN
|
Metabolism
|
[8] |
|
Metallothionein-1A (MT1A)
|
DE5ME8A
|
MT1A_HUMAN
|
Metabolism
|
[8] |
|
Metallothionein-2A (MT2A)
|
DEFKGT7
|
MT2_HUMAN
|
Metabolism
|
[8] |
|
Myeloperoxidase (MPO)
|
DEA3U9Y
|
PERM_HUMAN
|
Metabolism
|
[8] |
|
Quinone reductase 1 (NQO1)
|
DENP5RY
|
NQO1_HUMAN
|
Metabolism
|
[8] |
|
Glutathione S-transferase mu-1 (GSTM1)
|
DEYZEJA
|
GSTM1_HUMAN
|
Metabolism
|
[8] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
| ⏷ Show the Full List of 7 DME(s) |
|
|
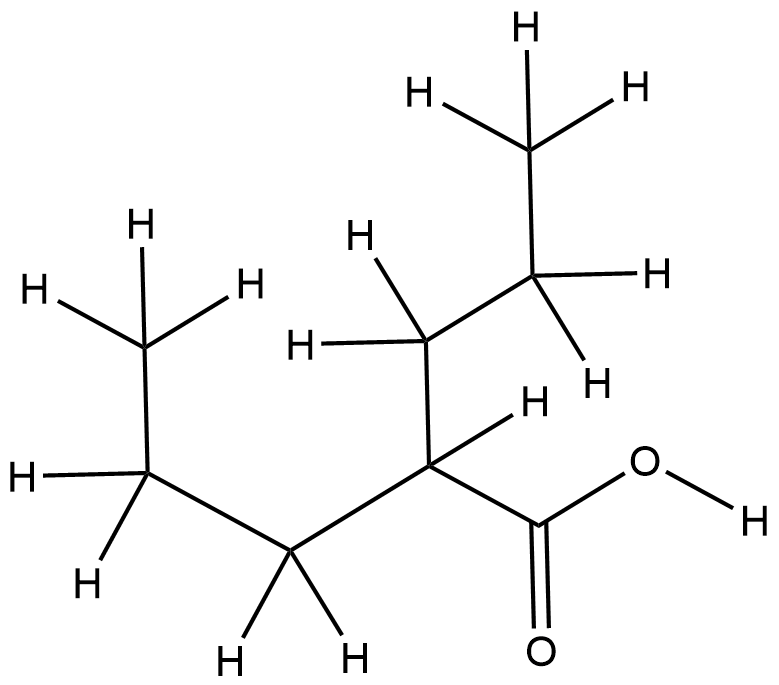
| Indication(s) of Valproic Acid |
| Disease Entry |
ICD 11 |
Status |
REF |
| Absence epilepsy |
N.A.
|
Approved |
[4] |
| Epilepsy |
8A60-8A68
|
Approved |
[4] |
| Glioblastoma |
2A00
|
Approved |
[4] |
| Coronavirus Disease 2019 (COVID-19) |
1D6Y
|
Investigative |
[5] |
| Neuroblastoma |
2D11.2
|
Investigative |
[4] |
|
|
|
|
|
|
|
|
|