| 1 |
ClinicalTrials.gov (NCT03782142) Effect on HIV Medications on EPC Cells
|
| 2 |
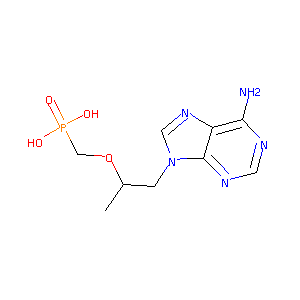
Tenofovir FDA Label
|
| 3 |
Anti-hepatitis B virus activity in vitro of combinations of tenofovir with nucleoside/nucleotide analogues. Antivir Chem Chemother. 2009;19(4):165-76.
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7365).
|
| 5 |
Antiviral drugs in current clinical use. J Clin Virol. 2004 Jun;30(2):115-33.
|
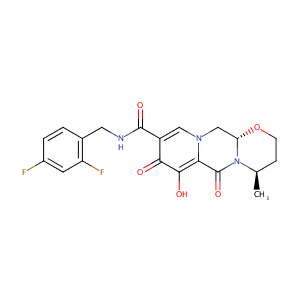
| 6 |
KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01665)
|
| 7 |
KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01913)
|
| 8 |
Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007 Feb;71(2):619-27.
|
| 9 |
Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids. 2001 Apr-Jul;20(4-7):641-8.
|
| 10 |
Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir Ther. 2014;19(7):687-92.
|
| 11 |
Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J Infect Dis. 2011 Jul 1;204(1):145-53.
|
| 12 |
Tenofovir Disoproxil Fumarate Is a New Substrate of ATP-Binding Cassette Subfamily C Member 11. Antimicrob Agents Chemother. 2017 Mar 24;61(4). pii: e01725-16.
|
| 13 |
Radium 223 dichloride for prostate cancer treatment. Drug Des Devel Ther. 2017 Sep 6;11:2643-2651.
|
| 14 |
Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition.
|
| 15 |
Mechanisms of action, pharmacology and interactions of dolutegravir. Enferm Infecc Microbiol Clin. 2015 Mar;33 Suppl 1:2-8.
|
| 16 |
Dolutegravir Impairs Stem Cell-Based 3D Morphogenesis Models in a Manner Dependent on Dose and Timing of Exposure: An Implication for Its Developmental Toxicity. Toxicol Sci. 2021 Nov 24;184(2):191-203. doi: 10.1093/toxsci/kfab112.
|
| 17 |
ClinicalTrials.gov (NCT03988452) Nucleosides And Darunavir/Dolutegravir In Africa
|
| 18 |
ClinicalTrials.gov (NCT02386098) Strategy-confirming Study of BMS-955176 to Treat HIV-1 Infected Treatment-experienced Adults
|
|
|
|
|
|
|