| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7945).
|
| 3 |
UBE2T promotes nasopharyngeal carcinoma cell proliferation, invasion, and metastasis by activating the AKT/GSK3/-catenin pathway.Oncotarget. 2016 Mar 22;7(12):15161-72.
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7884).
|
| 5 |
Chemerin promotes the pathogenesis of preeclampsia by activating CMKLR1/p-Akt/CEBP axis and inducing M1 macrophage polarization. Cell Biol Toxicol. 2022 Aug;38(4):611-628. doi: 10.1007/s10565-021-09636-7. Epub 2021 Aug 16.
|
| 6 |
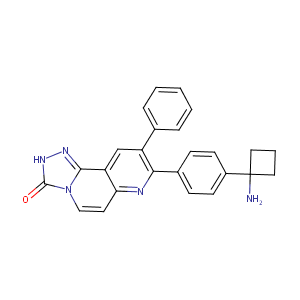
First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors.J Clin Oncol.2011 Dec 10;29(35):4688-95.
|
| 7 |
Platycodin D potentiates proliferation inhibition and apoptosis induction upon AKT inhibition via feedback blockade in non-small cell lung cancer cells. Sci Rep. 2016 Nov 29;6:37997. doi: 10.1038/srep37997.
|
| 8 |
Elucidating mechanisms of toxicity using phenotypic data from primary human cell systems--a chemical biology approach for thrombosis-related side effects. Int J Mol Sci. 2015 Jan 5;16(1):1008-29. doi: 10.3390/ijms16011008.
|
| 9 |
PI3K/AKT inhibitors aggravate death receptor-mediated hepatocyte apoptosis and liver injury. Toxicol Appl Pharmacol. 2019 Oct 15;381:114729. doi: 10.1016/j.taap.2019.114729. Epub 2019 Aug 22.
|
| 10 |
Harnessing the PI3K/Akt/mTOR pathway in T-cell acute lymphoblastic leukemia: eliminating activity by targeting at different levels. Oncotarget. 2012 Aug;3(8):811-23. doi: 10.18632/oncotarget.579.
|
| 11 |
-Mangostin alleviates liver fibrosis through Sirtuin 3-superoxide-high mobility group box 1 signaling axis. Toxicol Appl Pharmacol. 2019 Jan 15;363:142-153. doi: 10.1016/j.taap.2018.11.011. Epub 2018 Nov 29.
|
| 12 |
Akt activation by Ca(2+)/calmodulin-dependent protein kinase kinase 2 (CaMKK2) in ovarian cancer cells. J Biol Chem. 2017 Aug 25;292(34):14188-14204. doi: 10.1074/jbc.M117.778464. Epub 2017 Jun 20.
|
| 13 |
A novel c-Met inhibitor, MK8033, synergizes with carboplatin plus paclitaxel to inhibit ovarian cancer cell growth. Oncol Rep. 2013 May;29(5):2011-8.
|
| 14 |
Biologically active neutrophil chemokine pattern in tonsillitis.Clin Exp Immunol. 2004 Mar;135(3):511-8. doi: 10.1111/j.1365-2249.2003.02390.x.
|
|
|
|
|
|
|