Details of the Drug Combinations
General Information of This Drug (ID: DM1IS6U)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Apropovir; PMPA; TFV; Tenefovir; GS 1275; GS 1278; GS1278; GNA & Tenofovir; HHA & Tenofovir; KS-5021; Viread (TN); Viread, Tenofovir; D,L-Tenofovir; PMPA-(R); Phosphonic acid, [[2-(6-amino-9H-purin-9; [(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethylphosphonic acid; Phosphonic acid, [[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]-(9CI); Phosphonic acid, [[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]-& Galanthus nivalis agglutinin (GNA); Phosphonic acid, [[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]-& Hippeastrum hybrid agglutinin(HHA); (R)-9-(2-Phosphonomethoxypropyl)adenine; (R)-9-(2-Phosphonylmethoxypropyl)adenine; (R)-PMPA
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Anti-HIV Agents
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
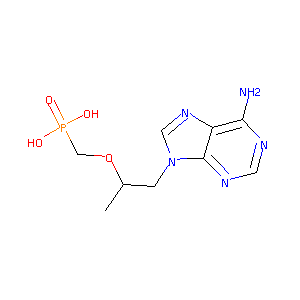 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
23 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
