| 1 |
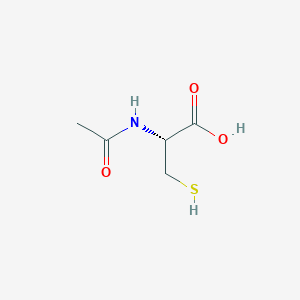
Acetylcysteine FDA Label
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 3 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 4 |
ClinicalTrials.gov (NCT04015557) Effect of Acetaminophen and N-Acetylcysteine on Liver Metabolism on Homocystinuria
|
| 5 |
ClinicalTrials.gov (NCT01187108) Study of Cobalt's Role in Excessive Erythrocytosis Among High Altitude Dwellers in Cerro de Pasco, Peru
|
| 6 |
ClinicalTrials.gov (NCT04545008) Trial of Famotidine & N-Acetyl Cysteine for Outpatients With COVID-19
|
| 7 |
ClinicalTrials.gov (NCT02269540) A New Treatment Approach for Major Depressive Disorder Based Upon Targeting Monoamine Oxidase A (MAO-A)
|
| 8 |
ClinicalTrials.gov (NCT02141620) n-Acetylcysteine and Cocaine
|
| 9 |
ClinicalTrials.gov (NCT06039280) Comparison of Dexamethasone and N Acetylcysteine (NAC) Versus N Acetylcysteine (NAC) Alone in the Prevention of Post Embolization Syndrome in Patients With Hepatocellular Carcinoma Following Transarterial Chemoembolization.
|
| 10 |
ClinicalTrials.gov (NCT03216954) Influence of n-Acetylcysteine Maintenance on Alcohol Effects
|
| 11 |
ClinicalTrials.gov (NCT00122018) An Investigation of N-acetylcysteine and Fenoldopam as Renal Protection Agents for Cardiac Surgery
|
| 12 |
ClinicalTrials.gov (NCT00558142) Mechanisms for the Effect of Acetylcysteine on Renal Function After Exposure to Radiographic Contrast Material
|
| 13 |
ClinicalTrials.gov (NCT01050270) Scottish and Newcastle Anti-emetic Pre-treatment for Paracetamol Poisoning Study (SNAP)
|
| 14 |
ClinicalTrials.gov (NCT02686385) Efficacy of N-acetylcysteine With or Without Steroids in Drug Induced Liver Injury
|
| 15 |
ClinicalTrials.gov (NCT04557215) Efficacy and Safety of Rifaximin With NAC in IBS-D
|
| 16 |
ClinicalTrials.gov (NCT02818270) Evaluation of Aerosolized Drugs Deposition During Mechanical Ventilation
|
| 17 |
ClinicalTrials.gov (NCT00497328) COmbined N-acetylcysteine and Bicarbonate in PCI To Reduce Adverse Side Effect of contrasT
|
| 18 |
ClinicalTrials.gov (NCT05126004) AZA Combined With NAC for PIT After HSCT
|
| 19 |
ClinicalTrials.gov (NCT02882477) Treatment of Wolfram Syndrome Type 2 With the Chelator Deferiprone and Incretin Based Therapy
|
| 20 |
ClinicalTrials.gov (NCT02707640) A Study to Assess the Safety and Tolerability of N-Acetylcysteine When Administered With Pirfenidone to Participants With Idiopathic Pulmonary Fibrosis (IPF)
|
| 21 |
ClinicalTrials.gov (NCT02723162) Translational Neuropsychopharmacology Research of Nicotine Addiction
|
| 22 |
ClinicalTrials.gov (NCT05070650) Efficacy and Safety of the Combination of Acetylcysteine, Paracetamol and Phenylephrine for the Treatment of Common Cold
|
| 23 |
ClinicalTrials.gov (NCT02750319) Amiodarone and N-Acetylcysteine or Amiodarone Alone for Preventing Atrial Fibrillation After Thoracic Surgery
|
| 24 |
ClinicalTrials.gov (NCT06139952) Ameliorating Contrast Induced Nephropathy After Coronary Angiography
|
|
|
|
|
|
|