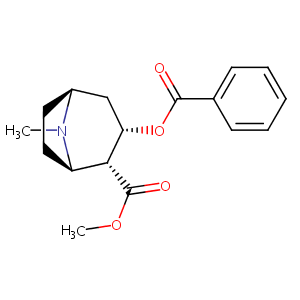
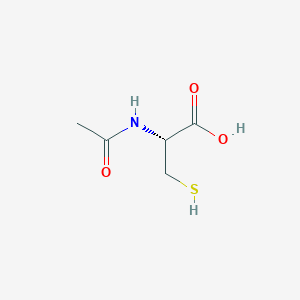
| DOT Name |
DOT ID |
UniProt ID |
Mode of Action |
REF |
|
Cholinesterase (BCHE)
|
OTOH3WQ9
|
CHLE_HUMAN
|
Increases Hydrolysis
|
[17] |
|
Alanine aminotransferase 1 (GPT)
|
OTOXOA0Q
|
ALAT1_HUMAN
|
Increases ADR
|
[18] |
|
Cocaine esterase (CES2)
|
OTC647SQ
|
EST2_HUMAN
|
Decreases Expression
|
[6] |
|
Cytoplasmic dynein 1 intermediate chain 1 (DYNC1I1)
|
OTFX1KCG
|
DC1I1_HUMAN
|
Decreases Expression
|
[6] |
|
Septin-4 (SEPTIN4)
|
OTD16B30
|
SEPT4_HUMAN
|
Decreases Expression
|
[6] |
|
LIM domain-binding protein 2 (LDB2)
|
OT6Y8IEK
|
LDB2_HUMAN
|
Decreases Expression
|
[6] |
|
Phospholipid phosphatase 2 (PLPP2)
|
OT0BQAXX
|
PLPP2_HUMAN
|
Decreases Expression
|
[6] |
|
Superoxide dismutase (SOD1)
|
OT39TA1L
|
SODC_HUMAN
|
Decreases Expression
|
[6] |
|
Protein kinase C beta type (PRKCB)
|
OTYQ0656
|
KPCB_HUMAN
|
Decreases Expression
|
[6] |
|
Gelsolin (GSN)
|
OT4KS2UU
|
GELS_HUMAN
|
Decreases Expression
|
[6] |
|
Neurofilament light polypeptide (NEFL)
|
OTQESJV4
|
NFL_HUMAN
|
Decreases Expression
|
[6] |
|
Neurofilament medium polypeptide (NEFM)
|
OT8VCBNF
|
NFM_HUMAN
|
Decreases Expression
|
[6] |
|
Profilin-1 (PFN1)
|
OTHTGA1H
|
PROF1_HUMAN
|
Increases Expression
|
[6] |
|
Cytochrome c1, heme protein, mitochondrial (CYC1)
|
OT0962IM
|
CY1_HUMAN
|
Decreases Expression
|
[6] |
|
Small ribosomal subunit protein uS2 (RPSA)
|
OTJZHEGT
|
RSSA_HUMAN
|
Decreases Expression
|
[6] |
|
Fibroblast growth factor 2 (FGF2)
|
OT7YUJ9F
|
FGF2_HUMAN
|
Decreases Expression
|
[6] |
|
Gamma-enolase (ENO2)
|
OTRODL0T
|
ENOG_HUMAN
|
Decreases Expression
|
[6] |
|
Guanine nucleotide-binding protein G(o) subunit alpha (GNAO1)
|
OTPB1RGK
|
GNAO_HUMAN
|
Decreases Expression
|
[6] |
|
Clathrin light chain B (CLTB)
|
OTPW9VRE
|
CLCB_HUMAN
|
Decreases Expression
|
[6] |
|
Cytochrome c oxidase subunit 6C (COX6C)
|
OT3V7JE5
|
COX6C_HUMAN
|
Decreases Expression
|
[6] |
|
Cadherin-1 (CDH1)
|
OTFJMXPM
|
CADH1_HUMAN
|
Decreases Expression
|
[6] |
|
Fos-related antigen 1 (FOSL1)
|
OT9YTYMB
|
FOSL1_HUMAN
|
Decreases Expression
|
[6] |
|
Neuroendocrine convertase 2 (PCSK2)
|
OT9Y2Z9B
|
NEC2_HUMAN
|
Decreases Expression
|
[6] |
|
Neuromodulin (GAP43)
|
OT2OTGGV
|
NEUM_HUMAN
|
Decreases Expression
|
[6] |
|
Receptor-type tyrosine-protein phosphatase alpha (PTPRA)
|
OTZA82J1
|
PTPRA_HUMAN
|
Decreases Expression
|
[6] |
|
Pleiotrophin (PTN)
|
OTYC7M2A
|
PTN_HUMAN
|
Decreases Expression
|
[6] |
|
Mitogen-activated protein kinase 3 (MAPK3)
|
OTCYKGKO
|
MK03_HUMAN
|
Increases Expression
|
[6] |
|
Gap junction beta-2 protein (GJB2)
|
OTBKLEYB
|
CXB2_HUMAN
|
Decreases Expression
|
[6] |
|
Malate dehydrogenase, cytoplasmic (MDH1)
|
OTJEO4E8
|
MDHC_HUMAN
|
Decreases Expression
|
[6] |
|
Vesicle-fusing ATPase (NSF)
|
OTKRJ2ZT
|
NSF_HUMAN
|
Decreases Expression
|
[6] |
|
Microtubule-associated protein 1B (MAP1B)
|
OTVXW089
|
MAP1B_HUMAN
|
Decreases Expression
|
[6] |
|
F-actin-capping protein subunit beta (CAPZB)
|
OTF1A4N0
|
CAPZB_HUMAN
|
Decreases Expression
|
[6] |
|
G protein-activated inward rectifier potassium channel 2 (KCNJ6)
|
OTDE1FT0
|
KCNJ6_HUMAN
|
Decreases Expression
|
[6] |
|
ATP synthase F(0) complex subunit C3, mitochondrial (ATP5MC3)
|
OTX674BU
|
AT5G3_HUMAN
|
Decreases Expression
|
[6] |
|
Caspase-4 (CASP4)
|
OTVQTV1L
|
CASP4_HUMAN
|
Decreases Expression
|
[6] |
|
Translocon-associated protein subunit delta (SSR4)
|
OTBWBGMR
|
SSRD_HUMAN
|
Decreases Expression
|
[6] |
|
Neuronal membrane glycoprotein M6-a (GPM6A)
|
OT8G13EG
|
GPM6A_HUMAN
|
Decreases Expression
|
[6] |
|
Mitogen-activated protein kinase 10 (MAPK10)
|
OTC46VX1
|
MK10_HUMAN
|
Decreases Expression
|
[6] |
|
Ataxin-3 (ATXN3)
|
OTJVVGKT
|
ATX3_HUMAN
|
Decreases Expression
|
[6] |
|
Myelin proteolipid protein (PLP1)
|
OT8CM9CX
|
MYPR_HUMAN
|
Decreases Expression
|
[6] |
|
Serine/threonine-protein phosphatase PP1-alpha catalytic subunit (PPP1CA)
|
OT7Y43A1
|
PP1A_HUMAN
|
Decreases Expression
|
[6] |
|
Serine/threonine-protein phosphatase 2A catalytic subunit beta isoform (PPP2CB)
|
OT24GMCM
|
PP2AB_HUMAN
|
Decreases Expression
|
[6] |
|
Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 (GNB1)
|
OTLL7L74
|
GBB1_HUMAN
|
Decreases Expression
|
[6] |
|
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform (PPP2CA)
|
OT83PT85
|
PP2AA_HUMAN
|
Decreases Expression
|
[6] |
|
Vacuolar fusion protein CCZ1 homolog B (CCZ1B)
|
OT1TTYK1
|
CCZ1B_HUMAN
|
Decreases Expression
|
[6] |
|
Interferon regulatory factor 9 (IRF9)
|
OTK4MYQJ
|
IRF9_HUMAN
|
Decreases Expression
|
[6] |
|
Transcription factor AP-4 (TFAP4)
|
OT1KQIUX
|
TFAP4_HUMAN
|
Increases Expression
|
[6] |
|
Dual specificity mitogen-activated protein kinase kinase 1 (MAP2K1)
|
OT4Y9NQI
|
MP2K1_HUMAN
|
Decreases Expression
|
[6] |
|
Focal adhesion kinase 1 (PTK2)
|
OT3Q1JDY
|
FAK1_HUMAN
|
Decreases Expression
|
[6] |
|
Paired box protein Pax-8 (PAX8)
|
OTRPD9MI
|
PAX8_HUMAN
|
Increases Expression
|
[6] |
|
Cadherin-17 (CDH17)
|
OT9EV2XK
|
CAD17_HUMAN
|
Decreases Expression
|
[6] |
|
Neuronal membrane glycoprotein M6-b (GPM6B)
|
OT8Q1582
|
GPM6B_HUMAN
|
Decreases Expression
|
[6] |
|
Hepatic sodium/bile acid cotransporter (SLC10A1)
|
OTUJVMCL
|
NTCP_HUMAN
|
Increases Expression
|
[6] |
|
Beta-sarcoglycan (SGCB)
|
OT5R5LAT
|
SGCB_HUMAN
|
Decreases Expression
|
[6] |
|
Hypoxia-inducible factor 1-alpha (HIF1A)
|
OTADSC03
|
HIF1A_HUMAN
|
Decreases Expression
|
[6] |
|
Rho GTPase-activating protein 29 (ARHGAP29)
|
OT8JH4TY
|
RHG29_HUMAN
|
Decreases Expression
|
[6] |
|
Glutamate decarboxylase 1 (GAD1)
|
OT52Z39J
|
DCE1_HUMAN
|
Decreases Expression
|
[6] |
|
Transgelin-3 (TAGLN3)
|
OTJBP3VJ
|
TAGL3_HUMAN
|
Decreases Expression
|
[6] |
|
Catenin delta-2 (CTNND2)
|
OTYKE30Y
|
CTND2_HUMAN
|
Decreases Expression
|
[6] |
|
Type 2 lactosamine alpha-2,3-sialyltransferase (ST3GAL6)
|
OTB17Q43
|
SIA10_HUMAN
|
Increases Expression
|
[6] |
|
Glucocorticoid receptor (NR3C1)
|
OTCI2YDI
|
GCR_HUMAN
|
Decreases Expression
|
[19] |
|
Cytochrome P450 2C8 (CYP2C8)
|
OTHCWT42
|
CP2C8_HUMAN
|
Decreases Expression
|
[19] |
|
Cytochrome P450 2C9 (CYP2C9)
|
OTGLBN29
|
CP2C9_HUMAN
|
Decreases Expression
|
[19] |
|
Nuclear receptor subfamily 1 group I member 3 (NR1I3)
|
OTS3SGH7
|
NR1I3_HUMAN
|
Decreases Expression
|
[19] |
|
Endothelin-1 receptor (EDNRA)
|
OTK9T5O3
|
EDNRA_HUMAN
|
Increases Expression
|
[20] |
|
Nitric oxide synthase 3 (NOS3)
|
OTLDT7NR
|
NOS3_HUMAN
|
Decreases Expression
|
[20] |
|
Chloride intracellular channel protein 1 (CLIC1)
|
OTYLFWNG
|
CLIC1_HUMAN
|
Decreases Expression
|
[21] |
|
Krev interaction trapped protein 1 (KRIT1)
|
OT58AP1I
|
KRIT1_HUMAN
|
Increases Expression
|
[21] |
|
Integrin beta-1-binding protein 1 (ITGB1BP1)
|
OTVQFNGS
|
ITBP1_HUMAN
|
Increases Expression
|
[9] |
|
5-hydroxymethyl-dUMP N-hydrolase (DNPH1)
|
OTEZVTI1
|
DNPH1_HUMAN
|
Decreases Expression
|
[9] |
|
Oligophrenin-1 (OPHN1)
|
OTDURZAV
|
OPHN1_HUMAN
|
Increases Expression
|
[21] |
|
Liprin-alpha-3 (PPFIA3)
|
OTNPZ6HT
|
LIPA3_HUMAN
|
Increases Expression
|
[22] |
|
NADH dehydrogenase iron-sulfur protein 2, mitochondrial (NDUFS2)
|
OTBT8KW9
|
NDUS2_HUMAN
|
Affects Expression
|
[23] |
|
Pituitary homeobox 3 (PITX3)
|
OTE2KT8P
|
PITX3_HUMAN
|
Decreases Expression
|
[24] |
|
Deformed epidermal autoregulatory factor 1 homolog (DEAF1)
|
OTCLX3ZW
|
DEAF1_HUMAN
|
Decreases Expression
|
[9] |
|
Claudin-11 (CLDN11)
|
OTNN6UTL
|
CLD11_HUMAN
|
Decreases Expression
|
[25] |
|
TIP41-like protein (TIPRL)
|
OTS2FZ8O
|
TIPRL_HUMAN
|
Increases Expression
|
[21] |
|
Protocadherin-8 (PCDH8)
|
OTDDOQM2
|
PCDH8_HUMAN
|
Increases Expression
|
[21] |
|
NADH dehydrogenase 1 alpha subcomplex subunit 10, mitochondrial (NDUFA10)
|
OTBURQ3A
|
NDUAA_HUMAN
|
Affects Expression
|
[23] |
|
Apoptosis-inducing factor 1, mitochondrial (AIFM1)
|
OTKPWB7Q
|
AIFM1_HUMAN
|
Increases Expression
|
[21] |
|
Reversion-inducing cysteine-rich protein with Kazal motifs (RECK)
|
OT9QIHEQ
|
RECK_HUMAN
|
Increases Expression
|
[21] |
|
Epidermal growth factor receptor (EGFR)
|
OTAPLO1S
|
EGFR_HUMAN
|
Decreases Expression
|
[21] |
|
Adenylate kinase isoenzyme 1 (AK1)
|
OT614AR3
|
KAD1_HUMAN
|
Decreases Expression
|
[9] |
|
Protein c-Fos (FOS)
|
OTJBUVWS
|
FOS_HUMAN
|
Increases Expression
|
[26] |
|
Beta-nerve growth factor (NGF)
|
OTOLABJT
|
NGF_HUMAN
|
Decreases Expression
|
[27] |
|
Natriuretic peptides A (NPPA)
|
OTMQNTNX
|
ANF_HUMAN
|
Increases Expression
|
[28] |
|
Pro-opiomelanocortin (POMC)
|
OTV41F7T
|
COLI_HUMAN
|
Increases Secretion
|
[29] |
|
Proenkephalin-A (PENK)
|
OT8P3HMP
|
PENK_HUMAN
|
Decreases Expression
|
[30] |
|
Proenkephalin-B (PDYN)
|
OTEJ6430
|
PDYN_HUMAN
|
Increases Expression
|
[9] |
|
Lutropin subunit beta (LHB)
|
OT5GBOVJ
|
LSHB_HUMAN
|
Increases Secretion
|
[31] |
|
Prolactin (PRL)
|
OTWFQGX7
|
PRL_HUMAN
|
Decreases Expression
|
[32] |
|
Insulin (INS)
|
OTZ85PDU
|
INS_HUMAN
|
Decreases Expression
|
[33] |
|
Tumor necrosis factor (TNF)
|
OT4IE164
|
TNFA_HUMAN
|
Decreases Expression
|
[34] |
|
Interferon gamma (IFNG)
|
OTXG9JM7
|
IFNG_HUMAN
|
Decreases Expression
|
[35] |
|
Interleukin-1 beta (IL1B)
|
OT0DWXXB
|
IL1B_HUMAN
|
Increases Expression
|
[8] |
|
Alpha-crystallin B chain (CRYAB)
|
OTY4JGYU
|
CRYAB_HUMAN
|
Decreases Expression
|
[9] |
|
Myelin basic protein (MBP)
|
OTFZCEDB
|
MBP_HUMAN
|
Decreases Expression
|
[9] |
|
C-reactive protein (CRP)
|
OT0RFT8F
|
CRP_HUMAN
|
Increases Expression
|
[36] |
|
Serum amyloid P-component (APCS)
|
OT76JZ8Z
|
SAMP_HUMAN
|
Increases Expression
|
[37] |
|
Platelet basic protein (PPBP)
|
OT1FHGQS
|
CXCL7_HUMAN
|
Increases Expression
|
[38] |
|
Platelet factor 4 (PF4)
|
OTEMJU68
|
PLF4_HUMAN
|
Increases Expression
|
[38] |
|
C-X-C motif chemokine 10 (CXCL10)
|
OTTLQ6S0
|
CXL10_HUMAN
|
Increases Secretion
|
[39] |
|
Transferrin receptor protein 1 (TFRC)
|
OT8ZPBDL
|
TFR1_HUMAN
|
Increases Expression
|
[9] |
|
Serotransferrin (TF)
|
OT41PEMS
|
TRFE_HUMAN
|
Decreases Expression
|
[25] |
|
Amyloid-beta precursor protein (APP)
|
OTKFD7R4
|
A4_HUMAN
|
Increases Expression
|
[9] |
|
Inhibin alpha chain (INHA)
|
OT7HWCO3
|
INHA_HUMAN
|
Decreases Expression
|
[21] |
|
Interleukin-5 (IL5)
|
OTAFPSCO
|
IL5_HUMAN
|
Increases Expression
|
[40] |
|
Interleukin-6 (IL6)
|
OTUOSCCU
|
IL6_HUMAN
|
Decreases Expression
|
[34] |
|
Endothelin-1 (EDN1)
|
OTZCACEG
|
EDN1_HUMAN
|
Increases Expression
|
[41] |
|
Transcription factor Jun (JUN)
|
OTCYBO6X
|
JUN_HUMAN
|
Increases Expression
|
[30] |
|
ATP synthase subunit beta, mitochondrial (ATP5F1B)
|
OTLFZUQK
|
ATPB_HUMAN
|
Decreases Expression
|
[22] |
|
Laminin subunit beta-1 (LAMB1)
|
OT6J9LJR
|
LAMB1_HUMAN
|
Increases Expression
|
[21] |
|
Calmodulin-1 (CALM2)
|
OTNYA92F
|
CALM1_HUMAN
|
Decreases Expression
|
[42] |
|
Interleukin-8 (CXCL8)
|
OTS7T5VH
|
IL8_HUMAN
|
Increases Secretion
|
[39] |
|
C-C motif chemokine 3 (CCL3)
|
OTW2H3ND
|
CCL3_HUMAN
|
Increases Secretion
|
[39] |
|
60 kDa heat shock protein, mitochondrial (HSPD1)
|
OTTO1Y11
|
CH60_HUMAN
|
Affects Expression
|
[23] |
|
Endoplasmic reticulum chaperone BiP (HSPA5)
|
OTFUIRAO
|
BIP_HUMAN
|
Increases Expression
|
[9] |
|
Serine/threonine-protein kinase pim-1 (PIM1)
|
OTWEKXTU
|
PIM1_HUMAN
|
Decreases Expression
|
[21] |
|
Angiotensin-converting enzyme (ACE)
|
OTDF1964
|
ACE_HUMAN
|
Increases Activity
|
[43] |
|
C-C motif chemokine 2 (CCL2)
|
OTAD2HEL
|
CCL2_HUMAN
|
Increases Secretion
|
[39] |
|
Glial fibrillary acidic protein (GFAP)
|
OTQ01ZAS
|
GFAP_HUMAN
|
Increases Expression
|
[30] |
|
D(2) dopamine receptor (DRD2)
|
OTBLXKEG
|
DRD2_HUMAN
|
Decreases Expression
|
[21] |
|
Ezrin (EZR)
|
OTHBPF1Z
|
EZRI_HUMAN
|
Decreases Expression
|
[8] |
|
Fos-related antigen 2 (FOSL2)
|
OT3MT9HJ
|
FOSL2_HUMAN
|
Decreases Expression
|
[30] |
|
Desmoplakin (DSP)
|
OTB2MOP8
|
DESP_HUMAN
|
Increases Expression
|
[22] |
|
CD44 antigen (CD44)
|
OT9TTJ41
|
CD44_HUMAN
|
Decreases Expression
|
[21] |
|
P-selectin (SELP)
|
OTWR90PK
|
LYAM3_HUMAN
|
Affects Localization
|
[38] |
|
Transcription factor JunB (JUNB)
|
OTG2JXV5
|
JUNB_HUMAN
|
Affects Expression
|
[30] |
|
Transcription factor JunD (JUND)
|
OTNKACJD
|
JUND_HUMAN
|
Decreases Expression
|
[30] |
|
Integrin beta-6 (ITGB6)
|
OTI3DJ7U
|
ITB6_HUMAN
|
Increases Expression
|
[21] |
|
Troponin I, cardiac muscle (TNNI3)
|
OT65E12V
|
TNNI3_HUMAN
|
Increases Secretion
|
[44] |
|
Cyclin-A2 (CCNA2)
|
OTPHHYZJ
|
CCNA2_HUMAN
|
Decreases Expression
|
[45] |
|
Parvalbumin alpha (PVALB)
|
OTZW1WVQ
|
PRVA_HUMAN
|
Decreases Expression
|
[22] |
|
Interleukin-10 (IL10)
|
OTIRFRXC
|
IL10_HUMAN
|
Decreases Secretion
|
[35] |
|
Nucleoside diphosphate kinase B (NME2)
|
OTCYGLHV
|
NDKB_HUMAN
|
Affects Expression
|
[23] |
|
Receptor-type tyrosine-protein phosphatase beta (PTPRB)
|
OTCQPLGZ
|
PTPRB_HUMAN
|
Decreases Expression
|
[21] |
|
Sodium-dependent noradrenaline transporter (SLC6A2)
|
OTMHQJMP
|
SC6A2_HUMAN
|
Decreases Activity
|
[46] |
|
Nuclear transcription factor Y subunit beta (NFYB)
|
OTXSE7KW
|
NFYB_HUMAN
|
Increases Expression
|
[21] |
|
Tumor necrosis factor receptor superfamily member 6 (FAS)
|
OTP9XG86
|
TNR6_HUMAN
|
Increases Expression
|
[47] |
|
ATP synthase subunit alpha, mitochondrial (ATP5F1A)
|
OT3FZDLX
|
ATPA_HUMAN
|
Affects Expression
|
[23] |
|
Elongation factor 1-gamma (EEF1G)
|
OTW7DH2F
|
EF1G_HUMAN
|
Decreases Expression
|
[9] |
|
CCN family member 2 (CCN2)
|
OTC39NSU
|
CCN2_HUMAN
|
Decreases Expression
|
[21] |
|
Transketolase (TKT)
|
OTT5KPUB
|
TKT_HUMAN
|
Affects Expression
|
[23] |
|
Peroxiredoxin-5, mitochondrial (PRDX5)
|
OTUEKZ18
|
PRDX5_HUMAN
|
Affects Expression
|
[23] |
|
Phosphatidylethanolamine-binding protein 1 (PEBP1)
|
OTQ5FEI0
|
PEBP1_HUMAN
|
Affects Expression
|
[23] |
|
CAP-Gly domain-containing linker protein 1 (CLIP1)
|
OTTGAEJE
|
CLIP1_HUMAN
|
Increases Expression
|
[21] |
|
Sodium-dependent serotonin transporter (SLC6A4)
|
OT6FGDLW
|
SC6A4_HUMAN
|
Decreases Activity
|
[48] |
|
Peroxiredoxin-2 (PRDX2)
|
OTLWCY9T
|
PRDX2_HUMAN
|
Decreases Expression
|
[22] |
|
Gastrin/cholecystokinin type B receptor (CCKBR)
|
OTQSNXOI
|
GASR_HUMAN
|
Decreases Expression
|
[21] |
|
D(3) dopamine receptor (DRD3)
|
OT0OFFKB
|
DRD3_HUMAN
|
Decreases Expression
|
[49] |
|
Alpha-actinin-2 (ACTN2)
|
OT9FOLD7
|
ACTN2_HUMAN
|
Increases Expression
|
[9] |
|
Hippocalcin-like protein 1 (HPCAL1)
|
OTOBBFRD
|
HPCL1_HUMAN
|
Decreases Expression
|
[9] |
|
Squalene synthase (FDFT1)
|
OTGDISIT
|
FDFT_HUMAN
|
Increases Expression
|
[42] |
|
Alpha-synuclein (SNCA)
|
OTPWC1MR
|
SYUA_HUMAN
|
Increases Expression
|
[50] |
|
Glutamate receptor 2 (GRIA2)
|
OT5N1WB7
|
GRIA2_HUMAN
|
Increases Expression
|
[51] |
|
Glutamate receptor 3 (GRIA3)
|
OT34CNBR
|
GRIA3_HUMAN
|
Increases Expression
|
[51] |
|
Caspase-3 (CASP3)
|
OTIJRBE7
|
CASP3_HUMAN
|
Increases Expression
|
[52] |
|
Beta-crystallin B2 (CRYBB2)
|
OTL0Z8E6
|
CRBB2_HUMAN
|
Decreases Expression
|
[9] |
|
Nuclear receptor subfamily 4 group A member 2 (NR4A2)
|
OT3F9IR2
|
NR4A2_HUMAN
|
Decreases Expression
|
[24] |
|
Small ribosomal subunit protein uS4 (RPS9)
|
OTPV69Q0
|
RS9_HUMAN
|
Decreases Expression
|
[9] |
|
Small ribosomal subunit protein eS10 (RPS10)
|
OTE3VSAH
|
RS10_HUMAN
|
Decreases Expression
|
[9] |
|
Protein Tob1 (TOB1)
|
OTNW949D
|
TOB1_HUMAN
|
Decreases Expression
|
[9] |
|
C-C chemokine receptor type 5 (CCR5)
|
OTP5FMZ4
|
CCR5_HUMAN
|
Increases Expression
|
[53] |
|
Protein FosB (FOSB)
|
OTW6C05J
|
FOSB_HUMAN
|
Increases Expression
|
[30] |
|
AP-2 complex subunit sigma (AP2S1)
|
OTEV2XGW
|
AP2S1_HUMAN
|
Decreases Expression
|
[9] |
|
Ephrin type-B receptor 4 (EPHB4)
|
OTKLPVXJ
|
EPHB4_HUMAN
|
Decreases Expression
|
[21] |
|
GTP-binding protein GEM (GEM)
|
OT6SK8ZV
|
GEM_HUMAN
|
Increases Expression
|
[9] |
|
Caspase-9 (CASP9)
|
OTD4RFFG
|
CASP9_HUMAN
|
Increases Expression
|
[52] |
|
C-X-C chemokine receptor type 4 (CXCR4)
|
OTUFSBX2
|
CXCR4_HUMAN
|
Increases Expression
|
[53] |
|
Eukaryotic translation initiation factor 5A-1 (EIF5A)
|
OTQ8DJX5
|
IF5A1_HUMAN
|
Decreases Expression
|
[47] |
|
Actin, gamma-enteric smooth muscle (ACTG2)
|
OTRDWUO0
|
ACTH_HUMAN
|
Decreases Expression
|
[8] |
|
Glutathione S-transferase omega-1 (GSTO1)
|
OTUJ93MN
|
GSTO1_HUMAN
|
Affects Expression
|
[23] |
|
Spectrin beta chain, non-erythrocytic 1 (SPTBN1)
|
OTOVM4D4
|
SPTB2_HUMAN
|
Decreases Expression
|
[9] |
|
Glutamate receptor ionotropic, NMDA 1 (GRIN1)
|
OTZ5YBO8
|
NMDZ1_HUMAN
|
Increases Expression
|
[51] |
|
Synaptic vesicular amine transporter (SLC18A2)
|
OTUOMMM6
|
VMAT2_HUMAN
|
Decreases Expression
|
[54] |
|
Apoptosis regulator BAX (BAX)
|
OTAW0V4V
|
BAX_HUMAN
|
Increases Expression
|
[8] |
|
Peptidyl-prolyl cis-trans isomerase D (PPID)
|
OT2CDF7W
|
PPID_HUMAN
|
Increases Expression
|
[21] |
|
Semaphorin-3B (SEMA3B)
|
OTCZCPMS
|
SEM3B_HUMAN
|
Increases Expression
|
[42] |
|
Dystrophin-related protein 2 (DRP2)
|
OTWR7WGU
|
DRP2_HUMAN
|
Affects Expression
|
[23] |
|
Calcium/calmodulin-dependent protein kinase type II subunit beta (CAMK2B)
|
OTS9YK3E
|
KCC2B_HUMAN
|
Decreases Expression
|
[42] |
|
Cullin-4B (CUL4B)
|
OT2QX4DO
|
CUL4B_HUMAN
|
Increases Expression
|
[9] |
|
Endoplasmic reticulum membrane sensor NFE2L1 (NFE2L1)
|
OT1QHOS2
|
NF2L1_HUMAN
|
Increases Expression
|
[21] |
|
Myocyte-specific enhancer factor 2D (MEF2D)
|
OT7CEIG0
|
MEF2D_HUMAN
|
Decreases Expression
|
[21] |
|
Proteasome activator complex subunit 4 (PSME4)
|
OTDGF53O
|
PSME4_HUMAN
|
Increases Expression
|
[9] |
|
Cysteine and glycine-rich protein 2 (CSRP2)
|
OT4SW0HI
|
CSRP2_HUMAN
|
Decreases Expression
|
[21] |
|
Cocaine- and amphetamine-regulated transcript protein (CARTPT)
|
OTTE4V9S
|
CART_HUMAN
|
Increases Expression
|
[9] |
|
BDNF/NT-3 growth factors receptor (NTRK2)
|
OTD557PU
|
NTRK2_HUMAN
|
Decreases Expression
|
[9] |
|
Prolyl endopeptidase-like (PREPL)
|
OTN5XMLZ
|
PPCEL_HUMAN
|
Increases Expression
|
[47] |
|
Myomegalin (PDE4DIP)
|
OTS15WNF
|
MYOME_HUMAN
|
Decreases Expression
|
[47] |
|
Adropin (ENHO)
|
OT91QASK
|
ENHO_HUMAN
|
Decreases Expression
|
[47] |
|
Liprin-beta-1 (PPFIBP1)
|
OTRA2S7B
|
LIPB1_HUMAN
|
Increases Expression
|
[21] |
|
Tumor protein p53-inducible nuclear protein 2 (TP53INP2)
|
OT0GTBXO
|
T53I2_HUMAN
|
Decreases Expression
|
[9] |
|
RING1 and YY1-binding protein (RYBP)
|
OTZZ4P2Z
|
RYBP_HUMAN
|
Increases Expression
|
[21] |
|
Programmed cell death protein 7 (PDCD7)
|
OTK5YO7J
|
PDCD7_HUMAN
|
Increases Expression
|
[21] |
|
Neuropilin and tolloid-like protein 2 (NETO2)
|
OT0YAMC0
|
NETO2_HUMAN
|
Increases Expression
|
[9] |
|
Scavenger receptor class B member 1 (SCARB1)
|
OTAE1UA1
|
SCRB1_HUMAN
|
Increases Expression
|
[42] |
|
Protein Churchill (CHURC1)
|
OTCVNVQN
|
CHUR_HUMAN
|
Decreases Expression
|
[9] |
|
Connector enhancer of kinase suppressor of ras 2 (CNKSR2)
|
OTAGERJ2
|
CNKR2_HUMAN
|
Increases Expression
|
[9] |
|
Lysophosphatidic acid receptor 1 (LPAR1)
|
OTJNJQPF
|
LPAR1_HUMAN
|
Decreases Expression
|
[9] |
|
Zinc finger protein ubi-d4 (DPF2)
|
OTL0JH2C
|
REQU_HUMAN
|
Increases Expression
|
[21] |
|
Tumor necrosis factor receptor superfamily member 14 (TNFRSF14)
|
OTB82PFO
|
TNR14_HUMAN
|
Increases Expression
|
[8] |
|
Protein MAL2 (MAL2)
|
OTVPEI80
|
MAL2_HUMAN
|
Increases Expression
|
[9] |
|
Protein TMEPAI (PMEPA1)
|
OTY8Z9UF
|
PMEPA_HUMAN
|
Increases Expression
|
[9] |
|
Serine/threonine-protein kinase Sgk3 (SGK3)
|
OTQ6QO99
|
SGK3_HUMAN
|
Increases Expression
|
[21] |
|
GRIP and coiled-coil domain-containing protein 1 (GCC1)
|
OTDOE6U8
|
GCC1_HUMAN
|
Increases Expression
|
[42] |
|
Fumarylacetoacetate hydrolase domain-containing protein 2A (FAHD2A)
|
OTHQP0L5
|
FAH2A_HUMAN
|
Affects Expression
|
[23] |
|
Protein arginine N-methyltransferase 6 (PRMT6)
|
OT5V3XIN
|
ANM6_HUMAN
|
Decreases Expression
|
[55] |
|
E3 ubiquitin-protein ligase NEDD4-like (NEDD4L)
|
OT1B19RU
|
NED4L_HUMAN
|
Increases Expression
|
[9] |
|
Eyes absent homolog 1 (EYA1)
|
OTHU807A
|
EYA1_HUMAN
|
Increases Expression
|
[9] |
|
Sigma non-opioid intracellular receptor 1 (SIGMAR1)
|
OTDORW5C
|
SGMR1_HUMAN
|
Affects Binding
|
[56] |
|
Apolipoprotein L2 (APOL2)
|
OT303Q7B
|
APOL2_HUMAN
|
Increases Expression
|
[42] |
|
Allograft inflammatory factor 1-like (AIF1L)
|
OTDEOB80
|
AIF1L_HUMAN
|
Decreases Expression
|
[9] |
|
Synaptotagmin-11 (SYT11)
|
OTLRTZEI
|
SYT11_HUMAN
|
Increases Expression
|
[21] |
|
Mitochondrial adenyl nucleotide antiporter SLC25A23 (SLC25A23)
|
OTG0B7RM
|
SCMC3_HUMAN
|
Decreases Expression
|
[47] |
|
Oxysterol-binding protein-related protein 1 (OSBPL1A)
|
OTQ33OQV
|
OSBL1_HUMAN
|
Decreases Expression
|
[9] |
|
Nuclear ubiquitous casein and cyclin-dependent kinase substrate 1 (NUCKS1)
|
OTL4VJC5
|
NUCKS_HUMAN
|
Decreases Expression
|
[9] |
|
Semaphorin-6A (SEMA6A)
|
OTDQ7QAW
|
SEM6A_HUMAN
|
Decreases Expression
|
[21] |
|
E3 ubiquitin-protein ligase MARCHF7 (MARCHF7)
|
OTG9SF10
|
MARH7_HUMAN
|
Increases Expression
|
[21] |
|
Serine/threonine-protein kinase PAK 6 (PAK6)
|
OTAHPZTT
|
PAK6_HUMAN
|
Decreases Expression
|
[21] |
|
Beta-catenin-interacting protein 1 (CTNNBIP1)
|
OTX9SBJG
|
CNBP1_HUMAN
|
Decreases Expression
|
[21] |
|
Protein lin-7 homolog C (LIN7C)
|
OTB3015O
|
LIN7C_HUMAN
|
Increases Expression
|
[21] |
|
Mitoferrin-1 (SLC25A37)
|
OTJ9F01S
|
MFRN1_HUMAN
|
Increases Expression
|
[9] |
|
Leucine-rich repeat transmembrane protein FLRT3 (FLRT3)
|
OT3XMZU3
|
FLRT3_HUMAN
|
Increases Expression
|
[21] |
|
Sodium/hydrogen exchanger 2 (SLC9A2)
|
OTYZYSHX
|
SL9A2_HUMAN
|
Decreases Expression
|
[21] |
|
Klotho (KL)
|
OTD4VWU6
|
KLOT_HUMAN
|
Increases Expression
|
[9] |
|
ProSAAS (PCSK1N)
|
OT7ZRW2F
|
PCS1N_HUMAN
|
Decreases Expression
|
[9] |
|
Protein FAM153A (FAM153A)
|
OTNHG4S2
|
F153A_HUMAN
|
Increases Expression
|
[47] |
|
Ectonucleotide pyrophosphatase/phosphodiesterase family member 5 (ENPP5)
|
OTY807N5
|
ENPP5_HUMAN
|
Increases Expression
|
[9] |
|
Probable ATP-dependent RNA helicase DDX41 (DDX41)
|
OT6KEIHP
|
DDX41_HUMAN
|
Decreases Expression
|
[21] |
|
Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 2 (HCN2)
|
OTW28QTH
|
HCN2_HUMAN
|
Increases Expression
|
[21] |
|
Protein NDRG2 (NDRG2)
|
OT5L6KD7
|
NDRG2_HUMAN
|
Decreases Expression
|
[9] |
|
SH3 and multiple ankyrin repeat domains protein 2 (SHANK2)
|
OTSQTPFQ
|
SHAN2_HUMAN
|
Decreases Expression
|
[21] |
|
Paraplegin (SPG7)
|
OT8OY9ST
|
SPG7_HUMAN
|
Decreases Expression
|
[21] |
|
Solute carrier family 22 member 14 (SLC22A14)
|
OTHRY1XS
|
S22AE_HUMAN
|
Decreases Expression
|
[21] |
|
UDP-N-acetylglucosamine transporter (SLC35A3)
|
OTG92DK5
|
S35A3_HUMAN
|
Increases Expression
|
[21] |
|
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B gamma isoform (PPP2R2C)
|
OTXK0SDM
|
2ABG_HUMAN
|
Decreases Expression
|
[9] |
|
Protocadherin gamma-A1 (PCDHGA1)
|
OTR1U1LI
|
PCDG1_HUMAN
|
Increases Expression
|
[21] |
|
Protocadherin alpha-2 (PCDHA2)
|
OTAV93M9
|
PCDA2_HUMAN
|
Increases Expression
|
[21] |
|
Mu-type opioid receptor (OPRM1)
|
OT16AAT8
|
OPRM_HUMAN
|
Increases Response To Substance
|
[57] |
|
Sodium-dependent dopamine transporter (SLC6A3)
|
OT39XG28
|
SC6A3_HUMAN
|
Increases Response To Substance
|
[58] |
|
Calcium/calmodulin-dependent protein kinase type IV (CAMK4)
|
OT47RDGV
|
KCC4_HUMAN
|
Increases Response To Substance
|
[59] |
|
Collagen alpha-1(XXI) chain (COL21A1)
|
OT7GS82E
|
COLA1_HUMAN
|
Affects Response To Substance
|
[10] |
|
Neuromedin-K receptor (TACR3)
|
OT7Q68XE
|
NK3R_HUMAN
|
Increases Response To Substance
|
[60] |
|
Liver carboxylesterase 1 (CES1)
|
OT9L0LR8
|
EST1_HUMAN
|
Increases Hydrolysis
|
[61] |
|
D(4) dopamine receptor (DRD4)
|
OTAJTO7N
|
DRD4_HUMAN
|
Increases Response To Substance
|
[62] |
|
Dopamine beta-hydroxylase (DBH)
|
OTC6I2SP
|
DOPO_HUMAN
|
Increases Response To Substance
|
[63] |
|
Cannabinoid receptor 1 (CNR1)
|
OTEALO6G
|
CNR1_HUMAN
|
Affects Response To Substance
|
[64] |
|
Synaptotagmin-13 (SYT13)
|
OTG94RJ4
|
SYT13_HUMAN
|
Affects Response To Substance
|
[10] |
|
Dynein axonemal heavy chain 8 (DNAH8)
|
OTGES2OU
|
DYH8_HUMAN
|
Affects Response To Substance
|
[10] |
|
Neuronal acetylcholine receptor subunit alpha-5 (CHRNA5)
|
OTGIL2YD
|
ACHA5_HUMAN
|
Decreases Response To Substance
|
[65] |
|
Little elongation complex subunit 2 (ICE2)
|
OTJ3BXND
|
ICE2_HUMAN
|
Affects Response To Substance
|
[10] |
|
Brain-derived neurotrophic factor (BDNF)
|
OTLGH7EW
|
BDNF_HUMAN
|
Decreases Response To Substance
|
[66] |
|
Glutathione S-transferase P (GSTP1)
|
OTLP0A0Y
|
GSTP1_HUMAN
|
Affects Response To Substance
|
[67] |
|
Voltage-dependent L-type calcium channel subunit alpha-1D (CACNA1D)
|
OTQFH2ZD
|
CAC1D_HUMAN
|
Affects Response To Substance
|
[68] |
|
CD81 antigen (CD81)
|
OTQFXNAZ
|
CD81_HUMAN
|
Increases Response To Substance
|
[69] |
|
Kelch-like protein 5 (KLHL5)
|
OTRDVST4
|
KLHL5_HUMAN
|
Affects Response To Substance
|
[10] |
|
Probable phospholipid-transporting ATPase IIB (ATP9B)
|
OTRTYQOK
|
ATP9B_HUMAN
|
Affects Response To Substance
|
[10] |
|
Glycoprotein endo-alpha-1,2-mannosidase (MANEA)
|
OTV7L8I6
|
MANEA_HUMAN
|
Affects Response To Substance
|
[10] |
|
Serine-rich coiled-coil domain-containing protein 1 (CCSER1)
|
OTVLD4J3
|
CCSE1_HUMAN
|
Affects Response To Substance
|
[10] |
|
Homer protein homolog 1 (HOMER1)
|
OTWFD3SI
|
HOME1_HUMAN
|
Increases Response To Substance
|
[70] |
|
Teneurin-3 (TENM3)
|
OTWY13GR
|
TEN3_HUMAN
|
Affects Response To Substance
|
[10] |
| ------------------------------------------------------------------------------------ |
|
|
|
|