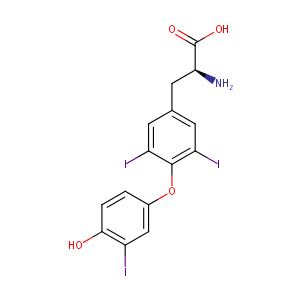
| Synonyms |
liothyronine; 3,3',5-Triiodo-L-thyronine; 6893/2/3; Liothyronin; Tresitope; 3,5,3'-triiodothyronine; L-Liothyronine; O-(4-Hydroxy-3-iodophenyl)-3,5-diiodo-L-tyrosine; triothyrone; Liothyroninum; Liotironina; 3,5,3'-Triiodo-L-thyronine; L-T3; T3; 3,3',5-Triiodothyronine; Triiodo-L-thyronine; Lyothyronine; 3,5,3'TRIIODOTHYRONINE; L-3,5,3'-Triiodothyronine; (2S)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]propanoic acid; T3 (amino acid); T3 (Hormone); L-3,3',5-TriioDOThyronine; Liothyronine [INN:BAN]; Rathyronine; Triothyrone; THYROID HORMONE; Liothyronine I 131; Liothyronine I 131 [USAN]; T3 liothyronine; Cytomel (TN); Euthroid-1; Euthroid-2; Euthroid-3; Liothyronine (INN); Liothyroninum[INN-Latin]; Liotironina [INN-Spanish]; T3 (Triiodothyronine); T3 (VAN); Tertroxin (TN); Thyrolar-1; Thyrolar-2; Thyrolar-3; Tri-Thyrotope; Triiodothyronine (T3); Triomet-131; Euthroid-05; Thyrolar-025; Thyrolar-05; TRIIODOTHYRONINE (T3 OR LIOTHYRONINE, ACTIVE) (6-11%); Thyronine, 3,3',5-triiodo-, L-(6CI); L-3-(4-(4-Hydroxy-3-iodophenoxy)-3,5-diiodophenyl)alanine; L-Tyrosine, O-(4-hydroxy-3-iodophenyl)-3,5-diiodo-(9CI); 3,3'5-Triiodo-L-thyronine; 3,5,3'-Tri-iodo-L-thyronine; 4-(3-Iodo-4-hydroxyphenoxy)-3,5-diiodophenylalanine; 4-(4-Hydroxy-3-iodophenoxy)-3,5-diiodophenylalanine; 4-(4-hydroxy-3-iodophenoxy)-3,5-diiodo-L-phenylalanine
|