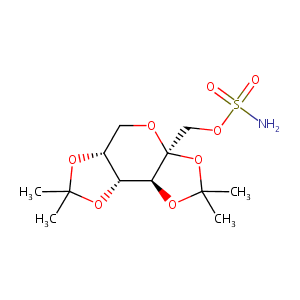
| Synonyms |
Epitoma; Epitomax; TOR; Tipiramate; Tipiramato; Topamac; Topamax; Topimax; Topina; Topiramato; Topiramatum; Topomax; Cilag brandof topiramate; Janssen brand of topiramate; Ortho brand of topiramate; Tipiramate [French]; Tipiramato [Spanish]; Topamax Sprinkle; Topiramate tablet; Topiramatum [Latin]; Topiramic acid; McN 4853; RWJ 17021; KS-1122; KW-6485; McN-4853; RWJ-17021; Topamax (TN); Topamax, Topiramate; Topiramate (TPM); Topiramate / Placebo; Topiramato [INN-Spanish]; Topiramatum [INN-Latin]; USL-255; RWJ-17021-000; Topiramate [USAN:BAN:INN]; Topiramate (JAN/USAN/INN); Beta-D-Fructopyranose, 2,3:4,5-bis-O-(1-methylethylidene)-, sulfamate; Beta-D-Fructopyranose, 2,3:4,5-bis-O-(1-methylethylidene)-, sulfamate (9CI);Beta.-D-Fructopyranose, 2,3:4,5-bis-O-(1-methylethylidene)-, 1-sulfamate; [(3aS,5aR,8aR,8bS)-2,2,7,7-tetramethyltetrahydro-3aH-bis[1,3]dioxolo[4,5-b:4',5'-d]pyran-3a-yl]methyl sulfamate; 2,3-4,5-bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate; 2,3:4,5-Bis-O-(1-methylethylidene) .beta.-D-fructopyranose sulfamate; 2,3:4,5-Bis-O-(1-methylethylidene)-36-D-fructo-pyranose sulfamate; 2,3:4,5-Bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate; 2,3:4,5-Di-O-isopropylidene-(beta)-D-fructopyranose sulfamate; 2,3:4,5-Di-O-isopropylidene-beta-D-fructopyranose sulfamate; 5H-Bis[1,3]dioxolo[4,5-b:4',5'-d]pyran, beta
|