Details of the Drug
General Information of Drug (ID: DM82Z30)
| Drug Name |
Topiramate
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Epitoma; Epitomax; TOR; Tipiramate; Tipiramato; Topamac; Topamax; Topimax; Topina; Topiramato; Topiramatum; Topomax; Cilag brandof topiramate; Janssen brand of topiramate; Ortho brand of topiramate; Tipiramate [French]; Tipiramato [Spanish]; Topamax Sprinkle; Topiramate tablet; Topiramatum [Latin]; Topiramic acid; McN 4853; RWJ 17021; KS-1122; KW-6485; McN-4853; RWJ-17021; Topamax (TN); Topamax, Topiramate; Topiramate (TPM); Topiramate / Placebo; Topiramato [INN-Spanish]; Topiramatum [INN-Latin]; USL-255; RWJ-17021-000; Topiramate [USAN:BAN:INN]; Topiramate (JAN/USAN/INN); Beta-D-Fructopyranose, 2,3:4,5-bis-O-(1-methylethylidene)-, sulfamate; Beta-D-Fructopyranose, 2,3:4,5-bis-O-(1-methylethylidene)-, sulfamate (9CI);Beta.-D-Fructopyranose, 2,3:4,5-bis-O-(1-methylethylidene)-, 1-sulfamate; [(3aS,5aR,8aR,8bS)-2,2,7,7-tetramethyltetrahydro-3aH-bis[1,3]dioxolo[4,5-b:4',5'-d]pyran-3a-yl]methyl sulfamate; 2,3-4,5-bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate; 2,3:4,5-Bis-O-(1-methylethylidene) .beta.-D-fructopyranose sulfamate; 2,3:4,5-Bis-O-(1-methylethylidene)-36-D-fructo-pyranose sulfamate; 2,3:4,5-Bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate; 2,3:4,5-Di-O-isopropylidene-(beta)-D-fructopyranose sulfamate; 2,3:4,5-Di-O-isopropylidene-beta-D-fructopyranose sulfamate; 5H-Bis[1,3]dioxolo[4,5-b:4',5'-d]pyran, beta
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticonvulsants
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
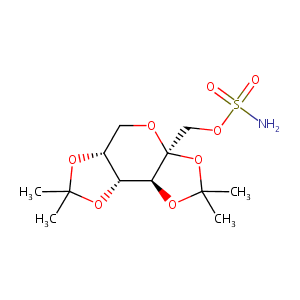 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 339.36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Topiramate (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6849). | ||||
|---|---|---|---|---|---|
| 2 | Topiramate FDA Label | ||||
| 3 | The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954. | ||||
| 4 | Knauf H, Mutschler E: Clinical pharmacokinetics and pharmacodynamics of torasemide. Clin Pharmacokinet. 1998 Jan;34(1):1-24. doi: 10.2165/00003088-199834010-00001. | ||||
| 5 | BDDCS applied to over 900 drugs | ||||
| 6 | Clinical pharmacology of topiramate: a review. Epilepsia. 2000;41 Suppl 1:S61-5. | ||||
| 7 | Walker MC, Sander JW: Topiramate: a new antiepileptic drug for refractory epilepsy. Seizure. 1996 Sep;5(3):199-203. | ||||
| 8 | Murrell GA, Rapeport WG: Clinical pharmacokinetics of allopurinol. Clin Pharmacokinet. 1986 Sep-Oct;11(5):343-53. doi: 10.2165/00003088-198611050-00001. | ||||
| 9 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 10 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 11 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 12 | Development of medications for alcohol use disorders: recent advances and ongoing challenges. Expert Opin Emerg Drugs. 2005 May;10(2):323-43. | ||||
| 13 | The antiepileptic drug topiramate is a substrate for human P-glycoprotein but not multidrug resistance proteins. Pharm Res. 2009 Nov;26(11):2464-70. | ||||
| 14 | Dose-dependent induction of cytochrome P450 (CYP) 3A4 and activation of pregnane X receptor by topiramate. Epilepsia. 2003 Dec;44(12):1521-8. | ||||
| 15 | Effects of anticonvulsants on human p450c17 (17alpha-hydroxylase/17,20 lyase) and 3beta-hydroxysteroid dehydrogenase type 2. Epilepsia. 2005 Mar;46(3):444-8. | ||||
| 16 | Design, synthesis, and biological evaluation of novel carbohydrate-based sulfamates as carbonic anhydrase inhibitors. J Med Chem. 2011 Mar 10;54(5):1481-9. | ||||
| 17 | A preliminary pharmacogenetic investigation of adverse events from topiramate in heavy drinkers. Exp Clin Psychopharmacol. 2009 Apr;17(2):122-9. doi: 10.1037/a0015700. | ||||
| 18 | Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3. | ||||
| 19 | Effects of the antiepileptic drugs lamotrigine, topiramate and gabapentin on hERG potassium currents. Epilepsy Res. 2005 Jan;63(1):17-25. doi: 10.1016/j.eplepsyres.2004.10.002. Epub 2004 Dec 8. | ||||
| 20 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 21 | Product Information. Topamax (topiramate). Ortho Pharmaceutical Corporation, Raritan, NJ. | ||||
| 22 | Mendelson J, Jones RT, Upton R, Jacob P 3rd "Methamphetamine and ethanol interactions in humans." Clin Pharmacol Ther 57 (1995): 559-68. [PMID: 7768079] | ||||
| 23 | Belcastro V, Costa C, Striano P "Levetiracetam-associated hyponatremia." Seizure 17 (2008): 389-90. [PMID: 18584781] | ||||
| 24 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 25 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 26 | Bourgeois BF "Drug interaction profile of topiramate." Epilepsia 37(suppl 2 (1996): s14-7. [PMID: 8641241] | ||||
| 27 | Product Information. Alphagan (brimonidine ophthalmic). Allergan Inc, Irvine, CA. | ||||
| 28 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 29 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 30 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 31 | Product Information. Thalomid (thalidomide). Celgene Corporation, Warren, NJ. | ||||
| 32 | Hansen BS, Dam M, Brandt J, et al "Influence of dextropropoxyphene on steady state serum levels and protein binding of three anti-epileptic drugs in man." Acta Neurol Scand 61 (1980): 357-67. [PMID: 6998251] | ||||
| 33 | Sekar M, Mimpriss TJ "Buprenorphine, benzodiazepines and prolonged respiratory depression." Anaesthesia 42 (1987): 567-8. [PMID: 3592200] | ||||
| 34 | Lambertsen CJ, Wendel H, Longenhagen JB "The separate and combined respiratory effects of chlorpromazine and meperidine in normal men controlled at 46 mm Hg alveolar pCO2." J Pharmacol Exp Ther 131 (1961): 381-93. [PMID: 13758472] | ||||
| 35 | Product Information. Zanaflex (tizanidine). Acorda Therapeutics, Hawthorne, NY. | ||||
| 36 | Product Information. Zyrtec (cetirizine). Pfizer US Pharmaceuticals, New York, NY. | ||||
