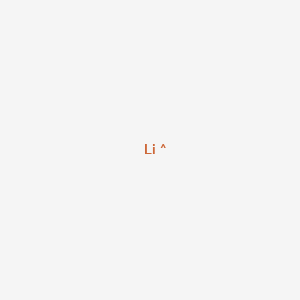| Synonyms |
7439-93-2; Li; litio; UNII-9FN79X2M3F; 9FN79X2M3F; CHEBI:30145; MFCD00134051; Litium; Lithium, metallic; Lithium, elemental; Lithium, 99+%, granular, dry; Lithium compounds; 3Li; HSDB 647; EINECS 231-102-5; UN1415; monolithium; Lithium standard solution, for AAS, 1 mg-ml Li in 2% HCl; Hydrure de lithium [French]; Lithium ribbon; Lithium rod; HSDB 549; Lithium granules; EINECS 231-484-3; UN1414; UN2805; Normothymin-E (TN); Epitope ID:114079; EC 231-102-5; Lithium, 99%, low sodium; Lithium, 99%, high sodium; CHEMBL2146126; DTXSID5036761; HSDB 6900; 7321AH; AKOS015833388; AKOS015902481; HSD6900000; Lithium wire, 3.2mm (0.125in) dia; Lithium [UN1415] [Dangerous when wet]; Lithium granules, 1-6mm (0.04-0.2in); Lithium, granular, 99% trace metals basis; FT-0627905; C15473; D08133; EC 231-484-3; Lithium hydride [UN1414] [Dangerous when wet]; Lithium, shot, 99%, 4-16 mesh, in mineral oil; Lithium, wire, diam. 3.2 mm, in mineral oil, >=98%; Lithium, ~25 wt % dispersion in mineral oil, high sodium; Lithium, AAS standard solution, Specpure?, Li 1000?g/ml; Lithium, ingot, diam. 5.7 cm, 99.9% trace metals basis; Lithium, rod, diam. 12.7 mm, 99.9% trace metals basis; Lithium foil, 0.75mm (0.03in) thick x 19mm (0.75in) wide; Lithium hydride, fused solid [UN2805] [Dangerous when wet]; Lithium ingot, 5.7cm (2.2in) dia x 8.6cm (3.4in) long; Lithium, Oil based standard solution, Specpure, Li 5000g/g; Lithium, plasma standard solution, Specpure?, Li 10,000?g/ml; Lithium, plasma standard solution, Specpure?, Li 1000?g/ml; Lithium, rod, 12.7 mm diameter, length 165 mm, purity 99%; Lithium, rod, 12.7 mm diameter, length 200 mm, purity 99%; Lithium, foil, 25x100mm, thickness 0.6mm, as rolled, 99.9%; Lithium, Oil based standard solution, Specpure(R), Li 1000?g/g; Lithium, foil, thickness 0.6 mm, size 25 x 300 mm, purity 99.9%; Lithium, Ion chromatography standard solution, Specpure, Li+ 1000?g/ml; Lithium, wire (in mineral oil), diam. 3.2 mm, 99.9% trace metals basis; Lithium, foil, not light tested, 38x200mm, thickness 0.20mm, as rolled, 99.9%; Lithium, foil, not light tested, 38x500mm, thickness 0.20mm, as rolled, 99.9%; Lithium, foil, not light tested, 45x200mm, thickness 0.12mm, as rolled, 99.9%; Lithium, granular, 4-10 mesh particle size, high sodium, 99% (metals basis); Lithium, ribbon, thickness x W 0.38 mm x 23 mm, 99.9% trace metals basis; Lithium, ribbon, thickness x W 0.75 mm x 19 mm, 99.9% trace metals basis; Lithium, ribbon, thickness x W 0.75 mm x 45 mm, 99.9% trace metals basis; Lithium, ribbon, thickness x W 1.5 mm x 100 mm, 99.9% trace metals basis
|